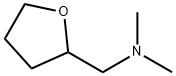
tetrahydrofurfuryl-N,N-dimethylamine synthesis
- Product Name:tetrahydrofurfuryl-N,N-dimethylamine
- CAS Number:30727-09-4
- Molecular formula:C7H15NO
- Molecular Weight:129.2
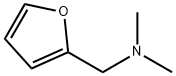
14496-34-5
19 suppliers
inquiry

30727-09-4
23 suppliers
$524.00/1g
Yield:30727-09-4 95%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in ethanol at 150; under 22502.3 Torr; for 5 h;Autoclave;Solvent;Pressure;Temperature;Reagent/catalyst;
Steps:
3 Example 3
50 g of furfural, 50 g of N, N-dimethylformamide (DMF) and 35 g of formic acid were added to a 500 mL round bottom flask. The flask was stirred in an oil bath while heating the reaction at a temperature of 130 ° C . Reaction time of 10 h. After the completion of the reaction, the residue was filtered off and the filtrate was distilled to recover formic acid and DMF. The residue was distilled under reduced pressure at 52.5 to 64 deg.C and 2000 Pa to obtain 56.7 g of N, N-dimethylfurfurylamine product with a molar yield of 86%. Then, 50.7 g of N,N-dimethylfurfurylamine product and 200 mL of ethanol were added to a 400 mL autoclave. 0.2 g of catalyst Pd/C was added, and the reaction was carried out at a temperature of 150 °C under hydrogen gas 3.0 MPa. The reaction time was 5h. After the end of the reaction, atmospheric distillation, recovery of solvent, collecting 155 ~ 162 ° C fractions N,N-dimethyltetrahydrofurfurylamine product 55.6g, hydrogenation molar yield of 95%, the total molar yield of products (based on wood Sugar) was 81.7%.
References:
CN106349195,2017,A Location in patent:Paragraph 0019-0042
109-99-9
1046 suppliers
$9.00/5ml

50-00-0
858 suppliers
$10.00/25g

124-40-3
509 suppliers
$18.00/100ml

30727-09-4
23 suppliers
$524.00/1g
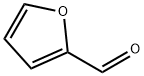
98-01-1
481 suppliers
$22.00/25g

30727-09-4
23 suppliers
$524.00/1g