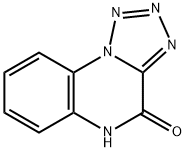
Tetrazolo[1,5-a]quinoxalin-4(5H)-one (8CI,9CI) synthesis
- Product Name:Tetrazolo[1,5-a]quinoxalin-4(5H)-one (8CI,9CI)
- CAS Number:29067-85-4
- Molecular formula:C8H5N5O
- Molecular Weight:187.16
![4-Chlorotetrazolo[1,5-a]quinoxaline](/CAS/GIF/59866-06-7.gif)
59866-06-7
13 suppliers
$200.00/50mg
![Tetrazolo[1,5-a]quinoxalin-4(5H)-one (8CI,9CI)](/CAS/GIF/29067-85-4.gif)
29067-85-4
18 suppliers
inquiry
Yield:29067-85-4 97%
Reaction Conditions:
with potassium hydroxide in lithium hydroxide monohydrate;(methylsulfinyl)methane at 25; for 0.75 h;
Steps:
5H-tetrazolo[1,5-a]quinoxalin-4-one (11h)
Name {P1|11h}: 5H-tetrazolo[1,5-a]quinoxalin-4-one; Formula: C8H5N5O; MolecularMass: 187.1582; Exact Mass: 187.0494; Smiles: O=c1[nH]c2ccccc2n2c1nnn2;InChIKey: SECOVEPJEIBBPV-UHFFFAOYSA-NThe starting material 4-chlorotetrazolo[1,5-a]quinoxaline (198 mg, 963 μmol, 1.00equiv) was dissolved in DMSO (6.00 mL); then 2 mL of water and potassium;hydroxide(273 mg, 4.86 mmol, 5.00 equiv) were added. The dark red reaction mixture wasstirred at 25 °C for 45 min, then a 1 M solution of HCl was added until the pH wasacidic. The precipitated product was collected via filtration and washed 3× with water; 5H-tetrazolo[1,5-a]quinoxalin-4-one (174 mg, 930 μmol, 97% yield) was obtained as alight yellow solid.Rf = 0.11 (cyclohexane/ethyl acetate 1:1). 1H NMR (400 MHz, DMSO-d6, ppm) δ =12.56 (bs, 1H, NH), 8.26 (dd, 3J = 8.3 Hz, 4J = 1.4 Hz, 1H, CHar), 7.64-7.60 (m, 1H,CHar), 7.49 (dd, 3J = 8.3 Hz, 4J = 1.4 Hz, 1H, CHar), 7.46-7.42 (m, 1H, CHar); 13C NMR(100 MHz, DMSO-d6, ppm) δ = 151.2 (1C, NCO), 144.4 (1C, NCN), 129.9 (1C, CHar),129.6 (1C, Cq), 124.0 (1C, CHar), 120.0 (1C, Cq), 116.9 (1C, CHar), 116.5 (1C, CHar);MS (ESI, negative Mode), m/z (%): 186 [M-1]- (100), 158 (34), 111 (27). HRMS (ESI,C8H5N5O): calcd 186.0415, found 186.0412; IR (ATR, ) = 3174 (w), 3104 (m), 3051(w), 3016 (w), 2959 (w), 2928 (w), 2898 (w), 2863 (w), 1704 (m), 1666 (vs), 1622 (s),1519 (m), 1472 (s), 1453 (s), 1418 (m), 1336 (vs), 1323 (s), 1264 (m), 1245 (s), 1210(s), 1156 (w), 1139 (w), 1113 (w), 1069 (m), 1017 (w), 993 (w), 943 (w), 864 (w), 792(m), 761 (vs), 728 (m), 704 (vs), 677 (vs), 652 (vs), 540 (w), 459 (vs), 446 (s) cm-1.Additional information on the chemical synthesis is available via Chemotion repository:https://doi.org/10.14272/reaction/SA-FUHFF-UHFFFADPSC-SECOVEPJEIUHFFFADPSC-NUHFF-NUHFF-NUHFF-ZZZAdditional information on the analysis of the target compound is available viaChemotion repository:https://doi.org/10.14272/SECOVEPJEIBBPV-UHFFFAOYSA-N.1The synthesis of this compound has been previously described and the 1H NMR datacorresponds with the literature [21].
References:
Holzhauer, Laura;Liagre, Chloé;Fuhr, Olaf;Jung, Nicole;Br?se, Stefan [Beilstein Journal of Organic Chemistry,2022,vol. 18,p. 1088 - 1099] Location in patent:supporting information
31595-63-8
23 suppliers
inquiry
![Tetrazolo[1,5-a]quinoxalin-4(5H)-one (8CI,9CI)](/CAS/GIF/29067-85-4.gif)
29067-85-4
18 suppliers
inquiry
59-48-3
459 suppliers
$5.00/1g
![Tetrazolo[1,5-a]quinoxalin-4(5H)-one (8CI,9CI)](/CAS/GIF/29067-85-4.gif)
29067-85-4
18 suppliers
inquiry
![Tetrazolo[1,5-a]quinoxaline](/CAS/20200401/GIF/235-27-8.gif)
235-27-8
0 suppliers
inquiry
![Tetrazolo[1,5-a]quinoxalin-4(5H)-one (8CI,9CI)](/CAS/GIF/29067-85-4.gif)
29067-85-4
18 suppliers
inquiry