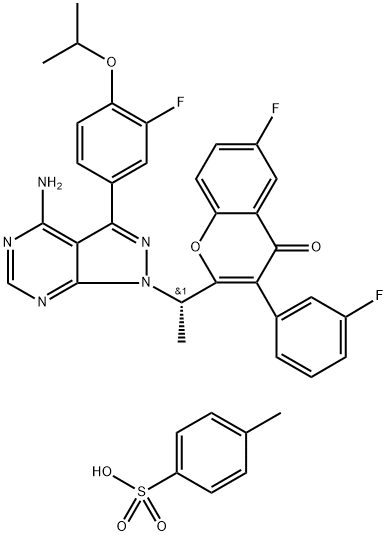
TGR-1202 (4-Methylbenzenesulfonate) synthesis
- Product Name:TGR-1202 (4-Methylbenzenesulfonate)
- CAS Number:1532533-72-4
- Molecular formula:C38H32F3N5O6S
- Molecular Weight:743.76
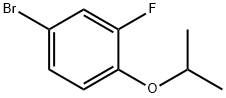
1532533-67-7
73 suppliers
inquiry
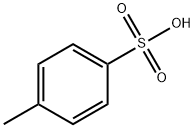
104-15-4
722 suppliers
$133.00/500g

1532533-72-4
2 suppliers
inquiry
Yield:1532533-72-4 95%
Reaction Conditions:
in isopropyl alcohol; for 1 h;Reflux;
Steps:
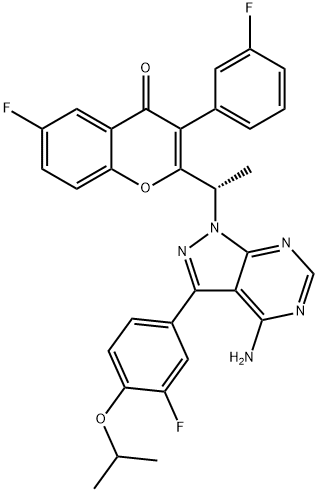
4-Methylbenzenesulfonate salt of Compound Bl (S)-2-(l-(4-amino-3-(3-fluoro-4-isopropoxyphenyl)-lH-pyrazolo[3,4-d]pyrimidin-l- yl)ethyl)-6-fluoro-3-(3-fluorophenyl)-4H-chromen-4-one 4-methylbenzenesulfonate
(S)-2-(l-(4-amino-3-(3-fluoro-4-isopropoxyphenyl)-lH^yrazolo[3,4-d]pyrimidin-l- yl)ethyl)-6-fluoro-3-(3-fluorophenyl)-4H-chromen-4-one 4-methylbenzenesulfonate: To (S)- 2-(l-(4-amino-3-(3-fluoro-4-isopropoxyphenyl)-lH-pyrazolo[3,4-d]pyrimidin-l-yl)ethyl)-6- fluoro-3-(3-fluorophenyl)-4H-chromen-4-one (22.7 g, 39.69 mmol) in isopropanol (600 ml), p-toluenesulphonic acid (8.30 g, 43.66 mmol) was added and refluxed for lh. The reaction mixture was concentrated, co-distilled with petroleum ether and dried. To the residue water (300 ml) was added and stirred for 30 min. The solid was filtered, washed with petroleum ether and dried under vacuum to afford the title compound as off-white solid (28.2 g, 95%). MP: 138-141°C. 'H-NMR (δ ppm, CDC13, 400 MHz): 8.11 (s, 1H), 7.85 (dd, J = 8.0,3.0 Hz, 1H), 7.80 (d, J = 8.2 Hz, 2H), 7.51 (dd, J = 9.3,4.3 Hz, 1H), 7.45 (dd, J = 7.5,3.1 Hz, 1H), 7.42-7.31 (m, 3H), 7.29 (m, 2H), 7.22 (d, J = 8.0 Hz, 2H), 7.16 (t, J = 8.3 Hz, 1H), 7.08 (dt, J = 8.5,2.5 Hz, 1H), 6.97 (br s, 1H), 6.88 (br s, 1H), 6.11 (q, J = 7.2 Hz, 1H), 4.67 (quintet, J = 6.0 Hz, 1H), 2.36 (s, 3H), 2.03 (d, J = 7.1Hz, 3H), 1.43 (d, J = 6.0 Hz, 6H). Mass : 572.4 (M+ + 1-PTSA). Enantiomeric excess: 93.4% as determined by HPLC on a chiralpak AD-H column, enriched in the fast eluting isomer (retention time = 12.35 min.)
References:
WO2014/6572,2014,A1 Location in patent:Page/Page column 129
202865-80-3
69 suppliers
$32.00/1g

1532533-72-4
2 suppliers
inquiry
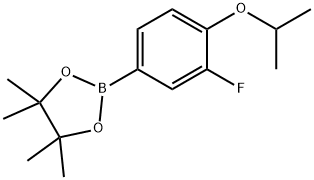
1350426-06-0
31 suppliers
inquiry

1532533-72-4
2 suppliers
inquiry
![3-(3-fluoro-4-isopropoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/20180529/GIF/1408087-64-8.gif)
1408087-64-8
24 suppliers
inquiry

1532533-72-4
2 suppliers
inquiry
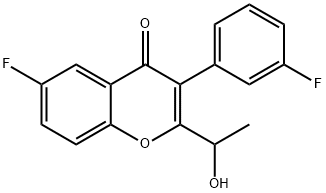
1532533-31-5
3 suppliers
inquiry

1532533-72-4
2 suppliers
inquiry