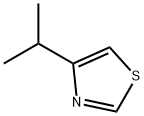
Thiazole, 4-(1-Methylethyl)- synthesis
- Product Name:Thiazole, 4-(1-Methylethyl)-
- CAS Number:17626-74-3
- Molecular formula:C6H9NS
- Molecular Weight:127.21
Yield:17626-74-3 70%
Reaction Conditions:
in 1,4-dioxane at 110; for 0.25 h;
Steps:
15
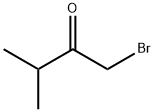
1-Bromo-3-methyl-butan-2-one (1.15 g, 6.95 mmol) and thioformamide ( 0.43 g, 6.95 mmol) were dissolved in dioxane (10 ml.) and heated in microwave at 110 °C for 15 min. Dichloromethane and NaHCO3 was added and after separation the organic phase was washed with NaOH (aq, 1 M) and water. Back-extracted water phase with dichloromethane. The combined organic phases were washed with water and brine, dried over Na2SO4, filtered and evaporated to yield 0.82g (70%) of 4- isopropyl-1 ,3-thiazole. EPO
References:
WO2006/64286,2006,A1 Location in patent:Page/Page column 121-122