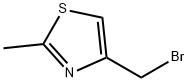
THIAZOLE, 4-(BROMOMETHYL)-2-METHYL- synthesis
- Product Name:THIAZOLE, 4-(BROMOMETHYL)-2-METHYL-
- CAS Number:74704-39-5
- Molecular formula:C5H6BrNS
- Molecular Weight:192.08
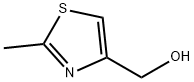
76632-23-0
87 suppliers
$26.00/250mg

74704-39-5
19 suppliers
inquiry
Yield:74704-39-5 61%
Reaction Conditions:
with N-Bromosuccinimide;triphenylphosphine in dichloromethane at -78; for 5 h;
Steps:
3 4-(Bromomethyl)-2-methyl-l,3-thiazole (S77):
To a stirred solution of hydro xymethyl thiazole S76 (0.910 g, 7.04 mmol, 1.0 equiv) in dichloromethane (20 mL) at -78 °C was added triphenylphosphine (1.85 g, 7.04 mmol, 1.0 equiv), followed by -bromo- succinimide (1.25 g, 7.04 mmol, 1.0 equiv). After 5 min, the reaction mixture was quenched with water (20 mL), and allowed to warm to 25 °C. The two phases were separated, and the aqueous layer was extracted with ethyl acetate (3 x20 mL). The combined organic layers were dried with anhydrous sodium sulfate and concentrated under reduced pressure. The obtained residue was purified by flash column chromatography (silica gel, 15- > 30% ethyl acetate in hexanes) to afford pure bromomethyl thiazole S778 (0.820 g, 4.27 mmol, 61% yield) as a colorless oil. S77: R{ =0.38 (silica gel, 30% ethyl acetate in hexanes); FT-IR (neat) v 3413, 3106, 2962, 2922, 2849, 1517, 1485, 1423, 1375, 1324, 1214, 1183, 1144, 1108, 1008, 955, 881, 853, 754, 702 cm"1; 'H NMR (600 MHz, CDC13) 5 = 7.13 (s, 1 H), 4.54 (s, 2 H), 2.72 (s, 3 H) ppm; 13C NMR (151 MHz, CDCI3) δ= 167.2, 151.7, 117.4, 27.2, 19.4 ppm.
References:
WO2018/191394,2018,A1 Location in patent:Page/Page column 182
6436-59-5
181 suppliers
$9.00/1g

74704-39-5
19 suppliers
inquiry
6436-60-8
41 suppliers
$39.00/1g

74704-39-5
19 suppliers
inquiry