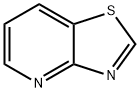
THIAZOLO[4,5-B]PYRIDINE synthesis
- Product Name:THIAZOLO[4,5-B]PYRIDINE
- CAS Number:273-98-3
- Molecular formula:C6H4N2S
- Molecular Weight:136.17
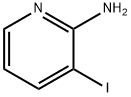
104830-06-0
230 suppliers
$7.00/250mg
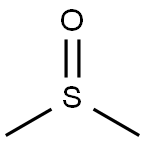
67-68-5
1214 suppliers
$15.00/100g
![THIAZOLO[4,5-B]PYRIDINE](/CAS/GIF/273-98-3.gif)
273-98-3
34 suppliers
inquiry
Yield:273-98-3 53%
Reaction Conditions:
with copper(l) iodide;potassium sulfide;ammonium acetate in water at 140; for 11 h;Schlenk technique;Inert atmosphere;
Steps:
13 Example 13
Add 2-amino-3-iodopyridine (0.2 mmol), potassium sulfide (0.6 mmol), dimethyl sulfoxide (2 mL), cuprous iodide (0.04 mmol), and ammonium acetate to the dry Schlenk reaction tube in sequence (1.2 mmol) and water (80 ul). After the sample is added, vacuum with an oil pump and inject nitrogen for gas replacement. After three replacements, the reaction is stopped at 140°C for 11 hours and then cooled to room temperature. The reaction is detected by thin-layer chromatography (TLC). After the reaction of the raw materials is completed, the reaction is terminated, and the mixed solution in the reaction tube is cooled to room temperature. Preliminary treatment of the mixed solution: passing through a short column, extracting, collecting the organic layer, spinning powder, and performing column chromatography to obtain the target product with a yield of 52%.
References:
CN111892553,2020,A Location in patent:Paragraph 0072-0074
![thiazolo[4,5-b]pyridin-2-amine](/CAS2/GIF/13575-41-2.gif)
13575-41-2
65 suppliers
$175.00/50mg
![THIAZOLO[4,5-B]PYRIDINE](/CAS/GIF/273-98-3.gif)
273-98-3
34 suppliers
inquiry
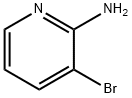
13534-99-1
358 suppliers
$4.00/1g
![THIAZOLO[4,5-B]PYRIDINE](/CAS/GIF/273-98-3.gif)
273-98-3
34 suppliers
inquiry