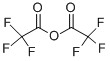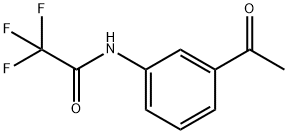
TIMTEC-BB SBB000702 synthesis
- Product Name:TIMTEC-BB SBB000702
- CAS Number:56915-87-8
- Molecular formula:C10H8F3NO2
- Molecular Weight:231.17
Yield:56915-87-8 100%
Reaction Conditions:
with pyridine in dichloromethane at 0; for 0.5 h;
Steps:
15
Example 15: General procedure for the preparation of N- methyl-enamine-sulfonamides of general formula (VII) following Scheme 4 N- (3-ACETYLPHENYL)-2, 2,2-trifluoroacetamide 5 g (37 mmol) of 3-aminoacetophenone were dissolved in 30 ml of anhydrous dichloromethane. To the resultant solution 3.15 ml (38.84 mmol) of anhydrous pyridine and 5.5 ml (38.84 mmol) of trifluoroacetic anhydride were added at 0°C. The reaction mixture was stirred for 30 minutes at the same temperature and poured onto 100 ml of water-ice. 100 ml of a saturated solution of sodium chloride were added and extracted with 2X70 ml of dichloromethane and 3X50 ml of ethyl acetate. The organic layers were washed with water, dried over anhydrous sodium sulfate and evaporated to dryness by reduced pressure distillation. 8. 7 g (yield= 100%) as a solid of N- (3-ACETYLPHENYL)-2, 2,2- trifluoroacetamide were obtained.
References:
WO2005/14597,2005,A1 Location in patent:Page/Page column 47; 48

431-47-0
273 suppliers
$10.00/5g
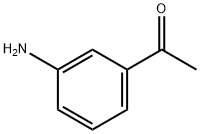
99-03-6
394 suppliers
$6.00/10g

56915-87-8
28 suppliers
$45.00/25mg
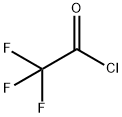
354-32-5
111 suppliers
$55.00/10g

99-03-6
394 suppliers
$6.00/10g

56915-87-8
28 suppliers
$45.00/25mg