TOPRAMEZONE synthesis
- Product Name:TOPRAMEZONE
- CAS Number:210631-68-8
- Molecular formula:C16H17N3O5S
- Molecular Weight:363.39
247922-29-8
6 suppliers
inquiry

2597-44-6
2 suppliers
inquiry
33641-15-5
197 suppliers
$12.00/1g

210631-68-8
63 suppliers
$255.00/100mg
Yield:210631-68-8 84.4%
Reaction Conditions:
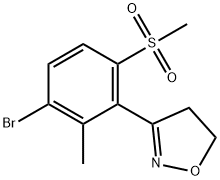
Stage #1:3-(3-bromo-2-methyl-6-(methylsulfonyl)phenyl)-4,5-dihydroisoxazole with potassium carbonate in 1,4-dioxaneReflux;
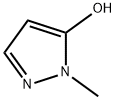
Stage #2:1-methyl-5-hydroxypyrazole with triphenylphosphine;sodium iodide in 1,4-dioxane at 60; for 0.5 h;
Stage #3:formyl radical with palladium on activated charcoal in 1,4-dioxane at 120; under 11251.1 Torr; for 20 h;Reagent/catalyst;Temperature;
Steps:
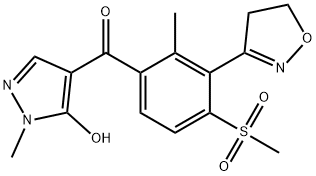
1; 2; 3-10 Example 1: Preparation of [3-(4,5-dihydroisoxazol-3-yl)-2-methyl-4-methylsulfonyl-phenyl]- (5-hydroxy-1-methyl-pyrazol-4-yl)methanone.
A round bottom flask (RBF) was charged with 22 gm (0.069 moles) of 3-(3-bromo-2-methyl- 6-methylsulfonyl-phenyl)-4,5-dihydroisoxazole followed by 100 gm of 1,4-dioxane and stirred it for 15 min. 17.6 gm (0.127 moles) of potassium carbonate was then charged in the round bottom flask. The reaction mixture was refluxed, and 50 gm of solvent was distilled off atmospherically. The slurry formed was then cooled to 25° C and transferred to clean pressure reactor, followed by addition of 6.8 gm (0.069 moles) of 2-methylpyrazol-3-ol, 2.07 gm (0.013 moles) of Sodium iodide, 0.72 gm (0.0027 moles) of Triphenylphosphine and 130 gm 1,4-dioxane. This reaction mixture was flushed with carbon monoxide gas, heated and stirred at 60° C for 30 min under carbon monoxide atmosphere. The reaction mixture was later cooled to 30° C. 0.88 gm (0.0003 moles) of Pd/C (RD-383) was added to the reaction mixture and the reaction mass was flushed with carbon monoxide gas and the reaction mixture was heated to 120° C under pressure of 15 bar of carbon monoxide gas for 20 hours. The reaction mass is then cooled to 60° C and the reactor was depressurized. 200 gm of water was added to the reaction mixture and was filtered through celite bed to remove catalyst and further the bed was washed with water. The filtrate was concentrated to remove 5 1,4-dioxane and water and the concentrated reaction mass was then diluted with 125 gm methanol. This reaction mixture was heated to 60° C and pH of reaction mixture was adjusted to 1.5 using 35percent hydrochloric acid. The reaction mixture was then cooled to 20° C within 3- 4 hours and the product was separated by filtration. The filtered product was then dried at 70-75° C under vacuum for 12 hours. The isolated product (i.e. [3-(4,5-dihydroisoxazol-3-yl)- 10 2-methyl-4-methylsulfonyl-phenyl]-(5-hydroxy-l-methyl-pyrazol-4-yl)methanone) had a yield of 84.4percent with purity of 99.37percent.
References:
BASF SE;YERANDE, Swapnil;SHINDE, Harish;BHOR, Vishal;KAPSE, Prashant;GOETZ, Roland WO2020/152200, 2020, A1 Location in patent:Page/Page column 30-31
223646-24-0
5 suppliers
inquiry

33641-15-5
197 suppliers
$12.00/1g

210631-68-8
63 suppliers
$255.00/100mg
123-91-1
664 suppliers
$16.00/25g

247922-29-8
6 suppliers
inquiry

33641-15-5
197 suppliers
$12.00/1g

210631-68-8
63 suppliers
$255.00/100mg

247922-29-8
6 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

33641-15-5
197 suppliers
$12.00/1g

210631-68-8
63 suppliers
$255.00/100mg
66794-10-3
15 suppliers
$285.00/1g

210631-68-8
63 suppliers
$255.00/100mg