
TRANS-2-(3,4,5-TRIFLUOROPHENYL)CYCLOPENTANOL synthesis
- Product Name:TRANS-2-(3,4,5-TRIFLUOROPHENYL)CYCLOPENTANOL
- CAS Number:1263869-56-2
- Molecular formula:C11H11F3O
- Molecular Weight:216.2

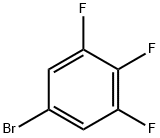
285-67-6
249 suppliers
$30.00/5g
138526-69-9
420 suppliers
$6.00/5g

1263869-56-2
5 suppliers
$437.00/1g
Yield:1263869-56-2 79%
Reaction Conditions:
Stage #1: 3,4,5-trifluoro-1-bromobenzenewith magnesium in tetrahydrofuran;Reflux;
Stage #2: Cyclopentene oxide;copper(l) iodide in tetrahydrofuran at 20; for 2 h;
Stage #3: with water;ammonium chloride in tetrahydrofuran;
Steps:
T.1
To a suspension of magnesium (2.88 g, 118 mmol) in THF (110 mL) was slowly added 5-bromo-l,2,3-trifluorobenzene (25 g, 118 mmol). After the addition, the reaction mixture was heated at reflux for 2 h. To this reaction mixture, copper (I) iodide (1.506 g, 7.91 mmol) and 6-oxabicyclo[3.1.0]hexane (9.93 g, 118 mmol) dissolved in THF (20 mL) were added dropwise. The reaction mixture warmed upon the addition of the epoxide. The reaction was stirred at rt for 2 h at rt. The reaction mixture was quenched by the addition of a solution of ammonium chloride. Ether was added and the organic layer was collected, dried (Na2SO4) and concentrated. The crude product was purified by column chromatography on silica gel to give (lS,2R)-2-(3,4,5-trifluorophenyl)cyclopentanol (20.2 g, 93 mmol, 79 % yield).1H NMR (500 MHz, CDCl3) δ ppm 6.79 - 7.02 (2H, m), 4.10 - 4.21 (IH, m), 2.75 - 2.91 (IH, m), 2.08 - 2.24 (2H, m), 1.75 - 1.93 (2H, m), 1.50 - 1.75 (2H, m).
References:
WO2011/14535,2011,A1 Location in patent:Page/Page column 134-135