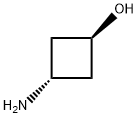
trans-3-Aminocyclobutanol synthesis
- Product Name:trans-3-Aminocyclobutanol
- CAS Number:1036260-45-3
- Molecular formula:C4H9NO
- Molecular Weight:87.12
![Carbamic acid, N-[trans-3-(phenylmethoxy)cyclobutyl]-, phenylmethyl ester](/CAS/20210305/GIF/937376-04-0.gif)
937376-04-0
0 suppliers
inquiry

1036260-45-3
38 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in acetic acid at 20 - 50; under 2585.81 Torr; for 4 - 6 h;
Steps:
4.6
A suspension of 4f (27 g) and Pd/C (3.9 g, 10% w/w) in acetic acid (163 mL) was shaken under ~50 psi for 1 hour at 200C and heated at 500C for ~3-5 hours. The reaction mixture was cooled to room temperature, filtered, washed with ethanol, and the filtrate concentrated until ~ 1 volume was left. The residue oil was dissolved in ethanol (55 mL) and triethylamine (55 mL). Di-t-butoxy dicarbonate (14.5g) was added, and the reaction mixture was stirred at room temperature over the weekend. The solution was concentrated to minimum volume. Water (110 mL) and methylene chloride (68 mL) and then saturated sodium bicarbonate (34 mL) were added. Two layers were separated. The aqueous layer was extracted with methylene chloride ( 2 X 68 mL) again. The combined organic layers were washed with brine (33 mL). The solution was then concentrated. Cyclohexane (108 mL) was added and then concentrated to 3.0 volumes. The solid was filtered, washed with cyclohexane (27ml) and the wet cake was dried under vacuum at 5O0C to provide 15.5 g of product, 4g, in a yield of 95%.
References:
WO2007/62333,2007,A2 Location in patent:Page/Page column 13