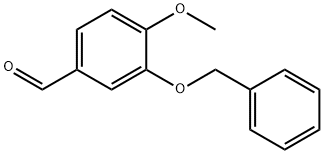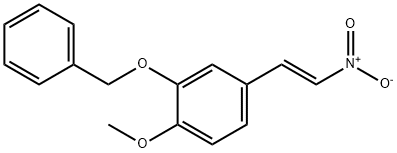
TRANS-3-BENZYLOXY-4-METHOXY-BETA-NITROSTYRENE synthesis
- Product Name:TRANS-3-BENZYLOXY-4-METHOXY-BETA-NITROSTYRENE
- CAS Number:63909-29-5
- Molecular formula:C16H15NO4
- Molecular Weight:285.29
Yield:63909-29-5 89.3%
Reaction Conditions:
Stage #1: 3-benzyloxy-4-methoxybenzaldehydewith ammonium acetate in acetic acid at 20; for 0.25 h;
Stage #2: nitromethane in acetic acid at 20 - 115; for 4.5 h;
Steps:
Example 2 Example 2: Preparation of (E)-2-(benzyloxy)-l-methoxy-4-(2-nitrovinyl)benzene (Compound 3)
[000117] A solution of compound 2 (385 g, 1.591 mmol, 1 eq) in acetic acid (2.5 L, 10 v), at rt, was added to ammonium acetate (306.5 g, 3.977 mmol, 2.5 eq), and stirred for 15 minutes. Nitromethane (255.5 mL, 4.773mmol, 3eq) was then added in drop wise manner at rt to this solution till the colour changes from colorless to light yellow. After completion of addition of nitromethane , the reaction mixture was stirred for 30 minutes at rt and heated to reflux (105- 115°C inner temperature). The reaction mixture was refluxed for 4 h, and filtered. The solid was washed with constant stirring in methanol for 30 min and filtered. The compound was then washed with pet ether (2x 1 L) dried. The final compound obtained was Compound 3 (405 g, 89.3%) as white solid (TLC system: 50% CHC13/DCM, Rf: 0.8). -NMR (400 MHz, DMSO-d6): 8.2 (d, J = 13.6Hz, 1H), 8.07 (d, J= 13.6Hz, 1H), 7.64 (d, J = 1.6Hz, 1H), 7.47-7.34 (m, 6H), 7.09 (d, J = 8.8Hz, 1H), 5.33 (s, 2H), 3.83 (s, 3H). LCMS purity: 99.43% at 214nm and 99.9% at 254nm (Zodiac C- 18 (150 x 4.6) mm, 3.5 micron, Mobile Phase A O.OlMaq. NH4OAc, B = CH3CN; Gradient (T/%B): 0/10, 10/90, 15/90, 15.1/10; Flow; 0.8ml/min, Rt= 10.89min; Diluent: CH3CN); Mass (m/z) = 284.1 (APCI, -ve mode).
References:
WO2016/51306,2016,A2 Location in patent:Paragraph 000117
621-59-0
639 suppliers
$9.00/1g

63909-29-5
55 suppliers
inquiry

100-39-0
435 suppliers
$10.00/10g

63909-29-5
55 suppliers
inquiry

100-44-7
658 suppliers
$13.50/250G

63909-29-5
55 suppliers
inquiry