
trans-4-(2-Propynyl)-cyclohexanemethanol synthesis
- Product Name:trans-4-(2-Propynyl)-cyclohexanemethanol
- CAS Number:250682-79-2
- Molecular formula:C10H16O
- Molecular Weight:152.23
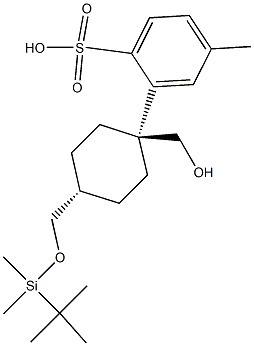
329788-70-7
3 suppliers
inquiry

250682-79-2
11 suppliers
$305.00/50mg
Yield:250682-79-2 93%
Reaction Conditions:
Stage #1: with ammonia;lithium in water at -78; for 1 h;
Stage #2: acetylene in dimethyl sulfoxide at 30; for 2 h;
Stage #3: ((1R,4R)-4-(((tert-butyldimethylsilyl)oxy)methyl)cyclohexyl)methyl 4-methylbenzenesulfonatewith tetrabutyl ammonium fluoridemore than 3 stages;
Steps:
3
A 3-neck 250 mL-flask equipped with a gas inlet tube and dry-ice condenser was cooled to -78° C. and charged with liquid ammonia (40 mL). To the reaction mixture was added lithium wire (600 mg, 86.4 mmol) generating a deep blue solution. The mixture was allowed to stir for 1 hour. Acetylene, passed through a charcoal drying tube, was added to the ammonia until all the lithium had reacted and the solution turned colorless, at which time the flow of acetylene was stopped, the acetylene-inlet tube and condenser removed and the flask outfitted with a thermometer. DMSO (20 mL) was added and the ammonia evaporated with a warm water bath until the mixture reached a temperature of 30° C. The solution was stirred at this temperature for 2 hours until the solution stopped bubbling. The mixture was cooled to 5° C. and compound 84 (11.25 g, 27.3 mmol), in DMSO (10 mL), was added. The temperature was maintained at 5° C. The mixture was allowed to stir at 5° C. for 0.5 hours. Then the solution was gradually warmed to room temperature and stirred for an additional 18 hours. The brown/black reaction mixture was poured slowly over ice (300 g) and extracted with ether (4×100 mL), dried with anhydrous sodium sulfate, and concentrated in vacuo to yield a yellow oil. The oil was subsequently dissolved in THF (200 mL) and changed to a brownish color upon addition of TBAF hydrate (11.20 g, 35.5 mmol). The solution was allowed to stir for 24 hours 2 under N2 atmosphere. After stirring, the reaction was quenched with water (200 mL) and extracted with ether (3×100 mL). The ether extracts were combined and concentrated in vacuo. The crude product was purified by chromatography, on a silica gel column, eluting with 1:1 ether/petroleum ether to yield 86 (3.91 g, 93%) as a yellow oil. 1H NMR (CDCl3) δ 3.45 (d, J=6.2, 2H), 2.10 (d, J=6.2, 2H), 1.9 (s, 1H), 1.94-1.69 (m, 4H), 1.52-1.34 (m, 2H), 1.16-0.83 (m, 4H); 13C NMR (CDCl3) δ 83.8, 69.5, 69.0, 40.8, 37.7, 32.3, 29.7, 26.5.
References:
US2006/40889,2006,A1 Location in patent:Page/Page column 24
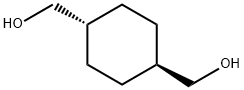
3236-48-4
173 suppliers
$7.00/1g

250682-79-2
11 suppliers
$305.00/50mg
![trans [4-(tert-Butyldimethylsilanyloxymethyl)-cyclohexyl]-methanol](/CAS/20200401/GIF/180046-62-2.gif)
180046-62-2
7 suppliers
inquiry

250682-79-2
11 suppliers
$305.00/50mg