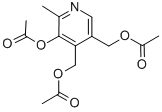
TRI-ACETYL PYRIDOXINE synthesis
- Product Name:TRI-ACETYL PYRIDOXINE
- CAS Number:10030-93-0
- Molecular formula:C14H17NO6
- Molecular Weight:295.29
Yield:10030-93-0 96%
Reaction Conditions:
with triethylamine in tert-butyl methyl ether at 50 - 65; for 2.5 h;Industry scale;Heating / reflux;
Steps:
1
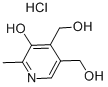
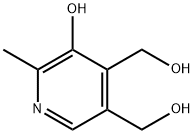
A 160 L reactor was inertised and charged with pyridoxine hydrochloride (II) (15.915 kg) in TBME (46 L). Acetic anhydride (3.25 eq. , 24.5 L) was added and the suspension was heated to 50°C. Triethylamine (4 eq. , 43 L) was added slowly over a period of 1 hour. During the addition of triethylamine, the inner temperature increased to 65°C. The mixture was stirred at reflux temperature for 1.5 hours. The reaction was completed when TLC analysis showed complete acetylation of pyridoxine. Workup The reaction was cooled to 10°C, filtered, and the precipitate was washed with TBME (3x 18 L). The combined organic layers were stirred for 25 minutes with saturated NaHCO3 (sat. 40 L soln. , pH 6-7). The organic and aqueous layers were separated, with the aqueous layer extracted again with TBME (20 L). The combined organic layers were extracted with saturated NaHC03 (40 L, pH 9). Analysis by'H NMR showed no residual acetic anhydride. The organic layer was stored in the reactor at 5°C overnight for the further work-up. The organic layer was extracted with brine (NaCl sat. soln. , 20 L) and the volume and LOD of the solution of acetylated product (C) in TBME were determined. LOD: 200 mL of the solution were evaporated to dryness to give 36.97 g of (C). This material still contained 1.08 % w/w of TBME (IH NMR). Yield for compound (C): 96%; purity 99.49% (by HPLC). In a second alternative and preferred workup, the reaction mixture (following complete conversion to compound (C) ) is cooled to 20°C and diluted with water (approximately 2. 80 L of water for every 1 kg of starting pyridoxine HC1). After phase separation the organic phase is washed with water. The combined aqueous phases are reextracted twice with TBME. The combined TBME phases are washed once with saturated NaHCO3-solution and once with diluted brine. The MTBE-product solution is concentrated to a concentration of about 50% and stored at room temperature until it is further converted. If this second alternative workup is used, the volume of MTBE in the synthetic step is preferably reduced by about 26 %. In a third alternative workup, the second alternative workup is followed, except that the step of washing the combined TBME phases with saturated NaHC03 solution is eliminated. NMR: Compound (C): 8 (ppm) = 8.45 (s, 1H, Harom) ; 5.27 (s, 2H, CH2) ; 5.14 (s, 2H, CH2) ; 2.42 (s, 3H, CH3) ; 2.39 (s, 3H, CH3), 2. 08 (s, 3H, CH3), 2.04 (s, 3H, CH3).
References:
WO2005/77902,2005,A2 Location in patent:Page/Page column 34-35