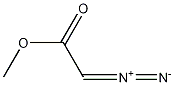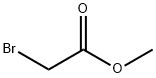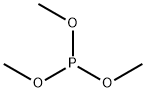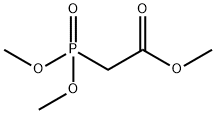
Trimethyl phosphonoacetate synthesis
- Product Name:Trimethyl phosphonoacetate
- CAS Number:5927-18-4
- Molecular formula:C5H11O5P
- Molecular Weight:182.11

96-34-4
335 suppliers
$10.00/25g
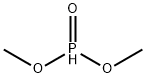
868-85-9
270 suppliers
$25.00/5 g

5927-18-4
320 suppliers
$5.00/25g
Yield:-
Reaction Conditions:
with potassium carbonate at 20 - 30;
Steps:
Synthesis of the composition
Methyl chloroacetate, 35.8 g (29.0 mL, 330 mmol), was added dropwise with vigorous stirring at 20-30° to a mixture of 36.6 g (30.4 mL, 330 mmol) of dimethyl phosphite and 91.0 g (660 mmol) of powdered K2CO3. The reaction mixture was stirred at 20-30° for 18 h, diluted with 50 mL of water, heated under reflux for 4 h, and let to cool down to room temperature. Concentrated HCl, 85 mL (990 mmol) , was then added with stirring, and the mixture was boiled for 6 h with distilletion of the water-metanol fraction up to bp 90°. The still residue at 20-30° was neutralized with 40% aqueous solution of 35.3 g (630 mmol) of KOH to 8, the mixture was stirred for 1 h at room temperature, and the precipitate of KCl that formed was filtered off to obtain 215.8 g of aqueous solution of di- and trisalts of [hydroxy(methoxy)phosphinoyl]-acetic (4) and phosphonoacetic (7, 8) acids (21% per phosphonoacetic acid, ratio 1 : 2), as well as phosphoric acid (1.6% per phosphoric acid) and KCl.
References:
Alimbarova;Kharlamov;Bondarenko;Barinskii [Russian Journal of General Chemistry,2015,vol. 85,# 10,p. 2441 - 2448][Ross. Khim. Zh.,2014,vol. 58,# 1,p. 23 - 30,8]

67-56-1
737 suppliers
$7.29/5ml-f
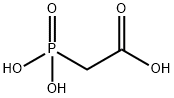
4408-78-0
88 suppliers
$45.00/1g

5927-18-4
320 suppliers
$5.00/25g