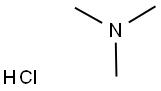
Trimethylamine hydrochloride synthesis
- Product Name:Trimethylamine hydrochloride
- CAS Number:593-81-7
- Molecular formula:C3H10ClN
- Molecular Weight:95.57
C3H9N+ HCl → C3H10ClN
The following steps are involved in obtaining trimethylamine hydrochloride:
Step 1: Evaporation of hydrochloric acid solution The hydrochloric acid solution is evaporated, first over a free flame, and later on a steam bath. This process results in concentration of the solution, which aids in the formation of trimethylamine hydrochloride crystals.
Step 2: Crystal formation and filtration As the solution becomes more and more concentrated, the trimethylamine hydrochloride crystals begin to form. The crystals are filtered periodically to remove them from the solution.
Step 3: Drying The filtered crystals are dried in an air bath for a few minutes, with a temperature range of 100-110°.
Step 4: Storage The air-dried crystals are then stored in a tightly closed bottle.
Step 5: Centrifugation (optional) If the trimethylamine hydrochloride crystals are centrifuged, the product is obtained pure and dry.
Overall, four runs yield an average of 710 g of pure trimethylamine hydrochloride and 82 g of slightly yellow-tinted product. This yield is equivalent to 89% of the theoretical amount based on ammonium chloride. The slight yellow coloration results from the evaporation of the last traces of solution.
DOI: 10.15227/orgsyn.001.0079
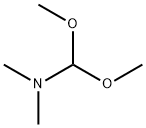
4637-24-5
639 suppliers
$5.00/25g
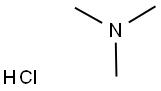
593-81-7
475 suppliers
$13.00/25g
Yield:593-81-7 96%
Reaction Conditions:
Stage #1: N,N-dimethyl-formamide dimethyl acetalwith [1,4-bis(diphenylphosphino)butane](1,5-cyclooctadiene)rhodium(I) tetrafluoroborate;hydrogen in methanol at 25; under 30003 Torr;Autoclave;
Stage #2: with hydrogenchloride in methanol;water;Reagent/catalyst;
Steps:
2-7 Examples 2-19
Examples 2-19 (0150) In a 10 ml autoclave, an amide acetal (5 mmol) is dissolved in 5 ml of absolute methanol, and, after adding 1 mol % catalyst, flushing with hydrogen is carried out. 40 bar of hydrogen are then injected in, and the mixture is stirred at 25° C. and a constant pressure until hydrogen absorption is no longer evident (1-2 h). After filtering off from the catalyst, the reaction solution is admixed with 10 ml of 1 M HCl solution in methanol, the solvent is removed on a rotary evaporator, and the residue is admixed with ether. The solid amine hydrochloride is filtered off, washed with diethyl ether and dried in vacuo.
References:
US2016/264513,2016,A1 Location in patent:Paragraph 0150; 0152

67-56-1
789 suppliers
$9.00/25ml
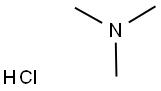
593-81-7
475 suppliers
$13.00/25g
75-50-3
285 suppliers
$15.00/25ml
593-81-7
475 suppliers
$13.00/25g

50-00-0
894 suppliers
$10.00/25g
593-81-7
475 suppliers
$13.00/25g

124-38-9
134 suppliers
$214.00/14L
593-81-7
475 suppliers
$13.00/25g