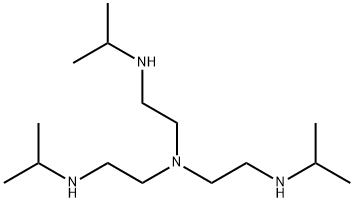
TRIS(2-(ISOPROPYLAMINO)ETHYL)AMINE synthesis
- Product Name:TRIS(2-(ISOPROPYLAMINO)ETHYL)AMINE
- CAS Number:157794-54-2
- Molecular formula:C15H36N4
- Molecular Weight:272.47

4097-89-6
200 suppliers
$10.00/1g

67-64-1
0 suppliers
$21.60/10ml

157794-54-2
15 suppliers
$160.00/5g
Yield:157794-54-2 72.4%
Reaction Conditions:
with sodium tetrahydroborate;sodium acetate;acetic acid in water at 0 - 20;
Steps:
4.1 2.4.1
Tris[2-(isopropylamino)-ethyl]amine (Isoprop3tren)
6?g (41.0?mmol) tris(2-aminoethyl)amine (tren), 50?mL (680.0?mmol) acetone, 90?mL water, 13?g sodium acetate and 42?mL acetic acid were mixed and cooled to 0?°C. 25?g sodium borohydride were (660.0?mmol) added over a time span of four hours.
The mixture was then warmed to room temperature and further stirred overnight.
The following day, sodium hydroxide was added until a pH of 12 was reached and two layers formed.
The aqueous layer was washed five times with 100?mL of diethyl ether.
The organic layers were combined and dried over magnesium sulfate.
Finally, the excess amount of solvent was removed by a rotary evaporator leading to a light yellow colored oil. Yield: 8.1?g (29.7?mmol, 72.4%).
1H NMR (CDCl3, 400?MHz): δ 1.3 (s, 3H, -CH), δ 2.4 (s, 12H, -CH2), δ 2.6 (m, 18H, -CH3). IR bands (cm-1): 3284?s (br), 2962 s, 2822 s, 1656 m, 1580 m, 1469 s, 1380 s, 1365 s, 1338 s, 1321 m, 1245 w, 1177 s, 1123 m, 1091 m, 1053 m, 955 w, 931 w, 852 w, 738 m, 485 w.
References:
Will, Janine;Würtele, Christian;Becker, Jonathan;Walter, Olaf;Schindler, Siegfried [Polyhedron,2019,vol. 171,p. 448 - 454]

4097-89-6
200 suppliers
$10.00/1g

67-64-1
0 suppliers
$21.60/10ml
![1,2-Ethanediamine, N1-(2-aminoethyl)-N2-(1-methylethyl)-N1-[2-[(1-methylethyl)amino]ethyl]-](/CAS/20210305/GIF/165329-31-7.gif)
165329-31-7
0 suppliers
inquiry

157794-54-2
15 suppliers
$160.00/5g