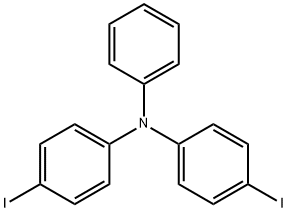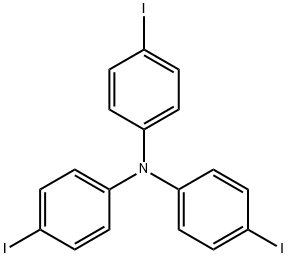
Tris(4-iodophenyl)amine synthesis
- Product Name:Tris(4-iodophenyl)amine
- CAS Number:4181-20-8
- Molecular formula:C18H12I3N
- Molecular Weight:623.01
603-34-9
312 suppliers
$24.89/5g

4181-20-8
197 suppliers
$9.00/1g
Yield:4181-20-8 99%
Reaction Conditions:
Stage #1:N,N-diphenylaminobenzene with trifluorormethanesulfonic acid;[bis(pyridine)iodine]+ tetrafluoroborate in dichloromethane at 0 - 20; for 21 h;
Stage #2: with water;sodium thiosulfate in dichloromethane
Steps:
4
Synthesis Example 4Tris(4-iodophenyl)amine was prepared as follows. Firstly, a mixture of IPy2BF4 (5.3 g, 14.3 mmol) and triphenylamine (1 g, 4.1 mmol) was added with dist. CH2Cl2 (60 mL) under a nitrogen atmosphere, and then was added dropwise at 0° C. with trifluoromethanesulfonic acid (TfOH: 900 μL, 4.1 mmol). Thereafter, the resultant mixture was stirred under a nitrogen atmosphere at room temperature for 21 hours to obtain a reddish-brown reaction mixture. Subsequently, the obtained reaction mixture was added with sat. Na2S2O3, and an aqueous layer wad extracted with CH2Cl2. After that, an organic layer thus collected was washed with sat. NaCl, and dried with Na2SO4. Subsequently, the dried organic layer was filtered and concentrated to obtain a crude product. Then, the obtained crude product was separated and purified by silica gel column chromatography (hexane/EtOAc=5/1) to obtain tris(4-iodophenyl) amine (2.507 g, 99% yield).The obtained compound was subjected to 1H NMR and 13C NMR measurements. The obtained results are shown below.1H NMR (CDCl3) δ7.54 (d, J=8.9 Hz, 6H), 6.81 (d, J=8.9 Hz, 6H).13C NMR (CDCl3) δ146.5, 138.4, 126.0, 86.6.From the NMR measurement results, it was confirmed that the obtained compound was tris(4-iodophenyl)amine.
References:
KABUSHIKI KAISHA TOYOTA CHUO KENKYUSHO;TOYOSHI SHIMADA US2008/227939, 2008, A1 Location in patent:Page/Page column 17
122-39-4
334 suppliers
$14.00/5g

4181-20-8
197 suppliers
$9.00/1g
108-86-1
497 suppliers
$10.00/5g

4181-20-8
197 suppliers
$9.00/1g