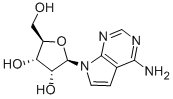
TUBERCIDIN synthesis
- Product Name:TUBERCIDIN
- CAS Number:69-33-0
- Molecular formula:C11H14N4O4
- Molecular Weight:266.25
![4-chloro-7-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-7H-pyrrolo[2,3-d]pyrimidine](/CAS/20210305/GIF/29914-75-8.gif)
29914-75-8
2 suppliers
inquiry

69-33-0
167 suppliers
$26.00/1mg
Yield:69-33-0 65 %
Reaction Conditions:
with ammonium hydroxide in 1,4-dioxane at 110;Sealed tube;Inert atmosphere;
Steps:
Synthesis of (2R,3R,4S,5R)-2-(4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (tubercidin) 15
The protected intermediate 14 (0.6 mmol, 1 eq.) was dissolved in 1,4-dioxane (4 mL), followed by theaddition of NH4OH (4 mL)). The reaction mixture was stirred at 110 °C in a sealed tube for 4 hours, thenconcentrated under vacuum. The crude residue was purified by automated flash column chromatographyeluting with DCM-MeOH 100:0 v/v increasing to DCM-MeOH 80:20 v/v in 15 CV to afford the titlecompound as an off-white solid in 65% yield. 1H-NMR (DMSO-d6), d: 8.03 (s, 1H), 7.32 (d, J= 3.6 Hz, 1H),7.04 (bs, 2H), 6.58 (d, J= 3.6 Hz, 1H), 5.97 (d, J=6.3 Hz, 1H, H-1’), 5.36-5.28 (m, 1H, 2’-OH), 5.28-5.20 (m, 1H,5’-OH), 5.08 (bs, 1H, 3’-OH), 4.43-4.38 (m, 1H, H-2’), 4.09-4.05 (m, 1H, H-3’), 3.88 (m, 1H, H-4’), 3.61 (m, 1H,H-5’), 3.55-3.48 (m, 1H, H-5’).
References:
Salerno, Martina;Varricchio, Carmine;Bevilacqua, Federica;Jochmans, Dirk;Neyts, Johan;Brancale, Andrea;Ferla, Salvatore;Bassetto, Marcella [European Journal of Medicinal Chemistry,2023,vol. 246,art. no. 114942] Location in patent:supporting information
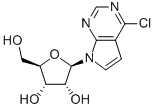
16754-80-6
75 suppliers
$55.00/100mg

69-33-0
167 suppliers
$26.00/1mg
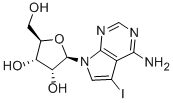
24386-93-4
173 suppliers
$13.00/2mg

69-33-0
167 suppliers
$26.00/1mg
![7H-Pyrrolo[2,3-d]pyrimidine, 4-(methylsulfonyl)-7-β-D-ribofuranosyl-](/CAS/20210305/GIF/120401-34-5.gif)
120401-34-5
0 suppliers
inquiry

69-33-0
167 suppliers
$26.00/1mg
![α-D-Ribofuranosyl chloride, 5-O-[(1,1-dimethylethyl)dimethylsilyl]-2,3-O-(1-methylethylidene)-](/CAS/20210305/GIF/102690-94-8.gif)
102690-94-8
1 suppliers
inquiry

69-33-0
167 suppliers
$26.00/1mg