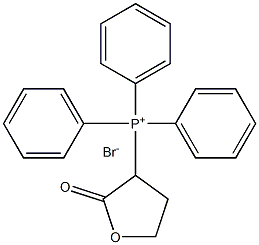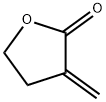
Tulipalin A synthesis
- Product Name:Tulipalin A
- CAS Number:547-65-9
- Molecular formula:C5H6O2
- Molecular Weight:98.1
![2(3H)-Furanone, 3-[(dimethylamino)methyl]dihydro-](/CAS/20210305/GIF/42023-17-6.gif)
42023-17-6
0 suppliers
inquiry
3140-73-6
470 suppliers
$9.00/25g

124-40-3
498 suppliers
$18.00/100ml

547-65-9
160 suppliers
$48.00/1g
13704-45-5
0 suppliers
inquiry
Yield:547-65-9 77% ,13704-45-5 94%
Reaction Conditions:
with triethylamine in isopropyl alcohol at 20; for 0.25 h;Eschenmoser Methylenation;
Steps:
synthesis of 3-methylenedihydrofuran-2(3H)-one (3c)
To a solution of a-dimethylaminomethyl-g-butyrolactone 1c (143 mg, 1.01 mmol) in 2-PrOH (5.0 mL) were added CDMT (176 mg, 1.01 mmol, 1.0 eq.) and Et3N (141 μL, 1.01 mmol, 1.0 eq.) at room temperature. After being stirred for 15 minutes, the reaction mixture was partitioned between Et2O and brine. The aqueous phase was extracted with Et2O three times and the combined organic phases were dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure and the residue was purified by flash column chromatography (hexane: dichloromethane: acetone = 10:1:1) to afford 3c (77.2 mg, 77 %), admixed with 4 (176.3 mg, 94 %). 1H NMR (CDCl3) d 6.27(m, 1H), 5.68(m, 1H), 4.38 (m, 2H), 2.99(m, 2H); 13C NMR (CDCl3) d 170.7, 133.4, 122.2, 65.2, 27.2; IR (CHCl3) cm-1: 2997, 2918, 1776, 1747, 1666, 1439, 1400, 1371, 1286, 1277, 1119, 1111, 1024, 945; LRMS (EI): 98 ([M]+ ).
References:
Yamada, Kohei;Masaki, Kazumasa;Hagimoto, Yuri;Kamiya, Seina;Kunishima, Munetaka [Tetrahedron Letters,2013,vol. 54,# 13,p. 1758 - 1760] Location in patent:supporting information
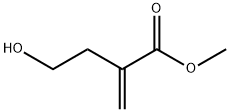
61541-20-6
0 suppliers
inquiry

547-65-9
160 suppliers
$48.00/1g
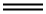
74-85-1
93 suppliers
$79.00/11l
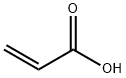
79-10-7
688 suppliers
$20.50/500ml

547-65-9
160 suppliers
$48.00/1g

2177-18-6
23 suppliers
$295.00/1mL