
TVDFOHQZQBXSFX-UHFFFAOYSA-N synthesis
- Product Name:TVDFOHQZQBXSFX-UHFFFAOYSA-N
- CAS Number:1392147-82-8
- Molecular formula:C11H12BrN
- Molecular Weight:238.12
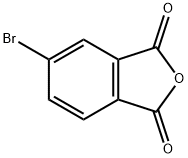
86-90-8
286 suppliers
$7.00/5g
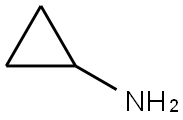
765-30-0
489 suppliers
$10.00/25g

1392147-82-8
16 suppliers
$525.00/1g
Yield:1392147-82-8 34%
Reaction Conditions:
Stage #1: 4-bromophthalic anhydride;Cyclopropylamine in toluene at 0 - 90; for 17 h;
Stage #2: with dimethylsulfide borane complex in tetrahydrofuran at 50; for 48 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran at 0 - 60; for 1 h;
Steps:
17
To a solution of phthalic anhydride (4.5 g, 20 mmol) in toluene (25 mL) was added cyclopropylannine (1 .52 mL) at 0 °C and the reaction mixture was stirred at 90 °C for 17 h. The solvent was evaporated and THF (20 mL) was added. To this was added BH3.Me2S THF complex 1 M (80 mL, 80 mmol) and the mixture was stirred at 50 °C for 48 h. The reaction was cooled to 0°C and poured onto a solution of 3 M HCI (27 mL) and stirred at 60 °C for 1 h. The mixture was washed with ethyl acetate, the aqueous phase was basified (pH 12) and extracted with DCM. The organic layer was dried, filtered and concentrated to afford the title compound as a yellow oil (1 .6 g, 34%). LCMS (ES+) 238, 240 (M+H)+.
References:
WO2012/103008,2012,A1 Location in patent:Page/Page column 96; 97