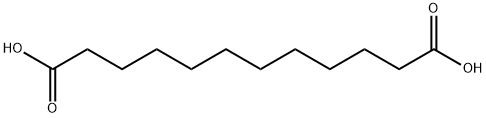
Undecanedioic acid synthesis
- Product Name:Undecanedioic acid
- CAS Number:1852-04-6
- Molecular formula:C11H20O4
- Molecular Weight:216.27
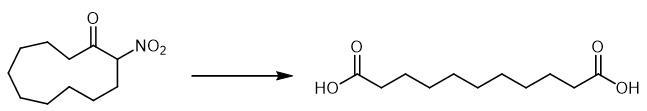
Fig The synthetic method 2 of Undecanedioic acid.

1092935-24-4
0 suppliers
inquiry

1852-04-6
285 suppliers
$5.00/10g
Yield:1852-04-6 100 %Chromat.
Reaction Conditions:
with hydrogen
Steps:
1
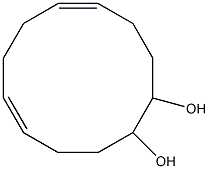
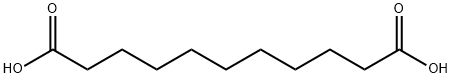
EXAMPLE 1; This example illustrates the synthesis of the C11 diacid starting from oleic acid. In a first stage, the ethenolysis of oleic acid is carried out at 30° C. in the presence of a tungsten-based catalyst in order to obtain 9-decenoic acid CH2CH-(CH2)7-COOH. For the second stage, use is made of the bispyridine ruthenium complex (8) catalyst described in the publication by Chen-Xi Bei et al., Tetrahedron Letters, 46 (2005), 7225-7228, in carrying out the cross-metathesis of 9-decenoic acid with methyl acrylate. The reaction is carried out in CH2Cl2, at a 0.1M 9-decenoic acid concentration and a 0.2M methyl acrylate concentration, at a temperature of 50° C. and for 12 hours. The yields are determined by chromatographic analysis. In the present case, use is made of 2 equivalents of methyl acrylate with respect to the acid and with a catalyst concentration of 0.5 mol %. The yield of product CH3-OOC-CHCH-(CH2)7-COOH is 50 mol %. This product can be hydrogenated according to a conventional process with a yield of 100%.
References:
US2010/305354,2010,A1 Location in patent:Page/Page column 6
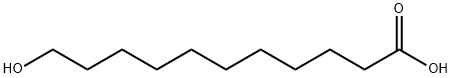
3669-80-5
69 suppliers
$104.00/100mg

1852-04-6
285 suppliers
$5.00/10g
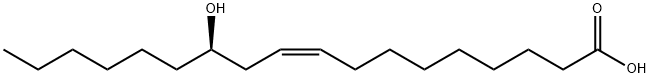
141-22-0
305 suppliers
$5.00/100g

1852-04-6
285 suppliers
$5.00/10g