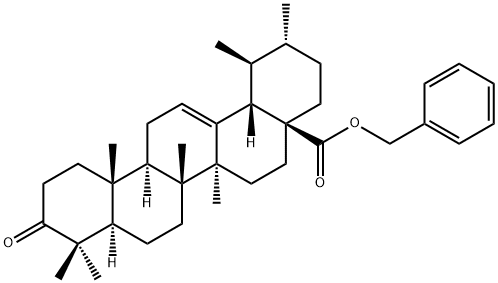
Ursonic acid benzyl ester synthesis
- Product Name:Ursonic acid benzyl ester
- CAS Number:869788-71-6
- Molecular formula:C37H52O3
- Molecular Weight:544.81
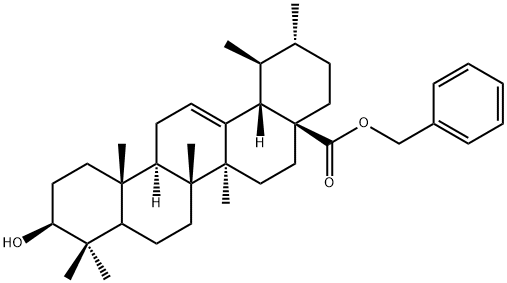
192211-41-9
5 suppliers
inquiry

869788-71-6
13 suppliers
inquiry
Yield:869788-71-6 95%
Reaction Conditions:
Stage #1: benzyl (1S,2R,4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,-11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylatewith oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -78 - -50; for 0.25 h;Inert atmosphere;Swern Oxidation;
Stage #2: with triethylamine in dichloromethane at -50 - 20;
Steps:
A2
To a solution of oxalyl chloride (2.57 mL, 5.14 mmol) in methylene chloride (5 mL) at -78°C under nitrogen was added dropwise a solution of DMSO (0.46 mL 6.4 mmol) in methylene chloride (5 mL). The mixture was allowed to warm to -50°C. To this was added a solution of the (l S,2R,4aS,6aS,6bR,8aR, 10S, 12aR, 12bR,14bS)-benzyl 10- hydroxy-l,2,6a,6b,9,9, 12a-heptamethyl- l,2,3,4,4a,5, 6,6a, 6b,7,8,8a,9, 10,1 1,12, 12a, 12b, 13, 14b-icosahydropicene-4a-carboxylate (intermediate Al) (2.34 gm, 4.28 mmol) in methylene chloride (15 mL) forming a white milky suspension. The mixture was stirred for an additional 15 minutes at -50°C after the addition, it was then treated with triethylamine (1.79 mL, 12.84 mmol) and the reaction mixture was slowly warmed to RT. It was diluted with methylene chloride (100 mL), washed with water (2 x 100 mL), followed by brine (50 mL). The organic phase was separated out, dried over anhydrous sodium sulfate, and concentrated in vacuo to a syrup. This crude material was partitioned over a silica gel column, eluted with 9: 1,hexanes: ethyl acetate solvent to give the title compound as a pale solid (2.22 g, 95%). XH NMR (400 MHz, CHLOROFORM-d) δ ppm 0.69 (s, 3 H), 0.87 (d, J=6.53 Hz, 3 H), 0.93 - 0.97 (m, 3 H), 1.03 (s, 3 H), 1.05 (s, 3 H), 1.09 (s, 6 H), 1.26 - 1.40 (m, 4 H), 1.40 - 1.54 (m, 5 H), 1.59 (d, J=9.03 Hz, 2 H), 1.70 (br. s., 2 H), 1.93 (dd, J=9.54, 3.26 Hz, 4 H), 1.97 - 2.08 (m, 2 H), 2.29 (d, J=11.04 Hz, 1 H), 2.38 (ddd, J=15.94, 6.90, 3.76 Hz, 1 H), 2.49 - 2.61 (m, 1 H), 4.97 - 5.03 (m, 1 H), 5.10 - 5.15 (m, 1 H), 5.27 (t, J=3.51 Hz, 1 H), 7.31 - 7.39 (m, 5 H).
References:
WO2011/153315,2011,A1 Location in patent:Page/Page column 112; 113
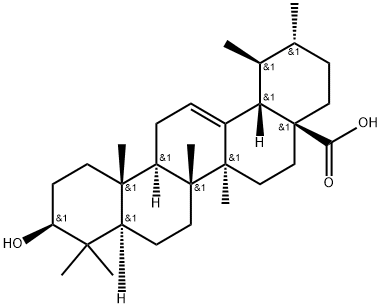
77-52-1
577 suppliers
$29.00/100mg

869788-71-6
13 suppliers
inquiry

100-39-0
425 suppliers
$10.00/10g

869788-71-6
13 suppliers
inquiry

100-44-7
630 suppliers
$13.50/250G

869788-71-6
13 suppliers
inquiry