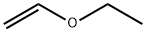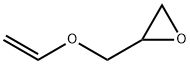
[(vinyloxy)methyl]oxirane synthesis
- Product Name:[(vinyloxy)methyl]oxirane
- CAS Number:3678-15-7
- Molecular formula:C5H8O2
- Molecular Weight:100.12
Yield:3678-15-7 63%
Reaction Conditions:
with 1,10-Phenanthroline;palladium diacetate in dichloromethane at 60; for 48 h;
Steps:
3.1 4.3.1. Vinyloxy-2-methyloxiranne (GcEV)
Palladium acetate (34.42 mg, 1.52 mmol) and 1,10 phenantroline (41.2 mg, 2.29 mmol) were dissolved separately in 10 mL of dichloromethane and mixed together at 20 °C for 15 min. 20.00 g (269.99 mmol) of glycidol and 263.00 g (2.29 mol) of vinyloxyethane were placed along with the catalyst solution into a pressure reactor. The reactor was closed and the reaction mixture was heated under stirring at 60 °C for 48 h. The volatiles were removed using a rotary evaporator. Diethyl ether was added to the residue, and the precipitated catalyst was filtered off. 17.03 g (170.09 mmol) of GcEV was then isolated by distillation under reduced pressure and obtained as a transparent colourless liquid. 1H NMR (Acetone-d6) δ (ppm): 0.89 (s, C-CH3, 6H), 2.15 (s, -CH2-N, 2H), 2.23 (s, N-CH3, 6H), 3.44 (s, -CH2-O, 2H), 3.91 (dd, CHH-CH=(E), 2Jgem = 1.77 Hz, 3Jcis = 6.82 Hz, 1H), 4.16 (dd, CHH-CH=(Z), 2Jgem = 1.64 Hz, 3Jtrans = 14.27 Hz, 1H), 6.51 (ddt, CH2-CH=, 3Jcis = 6.82 Hz, 3Jtrans = 14.27 Hz, 4J = 0.51 Hz, 1H). 13C NMR (Acetone-d6) δ (ppm): 23.5 (CH3-C, 2C), 37.1 ((CH3)2-C<, 1C), 48.8 (CH3-N, 2C), 67.2 (CH2-N, 1C), 74.7 (CH2-O, 1C), 86.2 (H2C=CH, 1C), 153.1 (H2C=CH, 1C). 1H NMR (Acetone-d6) δ (ppm): 2.61 (dd, -CHH-O- epoxy, 2Jgem = 5.05 Hz, 3Jtrans = 2.53, 1H), 2.77 (dd, -CHH-O- epoxy, 2Jgem = 5.05 Hz, 3Jcis = 4.17 Hz, 1H), 3.17 (m, -CH2-CH<, 1H), 3.56 (dd, -O-CHH-, 2Jgem = 11.62 Hz, 3Jcis = 6.32 Hz, 1H), 3.99 (dd, CHH-CH = (E), 2Jgem = 2.02 Hz, 3Jcis = 6.82 Hz, 1H), 4.01 (dd, -O-CHH-, 2Jgem = 11.62 Hz, 3Jtrans = 2.78 Hz, 1H), 4.22 (dd, CHH-CH = (Z), 2Jgem = 2.02 Hz, 3Jtrans = 14.27 Hz, 1H), 6.50 (ddt, CH2-CH=, 3Jcis = 6.82 Hz, 3Jtrans = 14.27 Hz, 4J = 0.51 Hz, 1H). 13C NMR (Acetone-d6) δ (ppm): 44.2 (CH-CH2-O-, 1C), 50.3 (-CH2-CH<, 1C), 70.1 (>HC-CH2-O-, 1C), 87.4 (H2C=CHO-, 1C), 152.6 (>C=CH2, 1C).
References:
Couture, Guillaume;Ladmiral, Vincent;Améduri, Bruno [Journal of Fluorine Chemistry,2015,vol. 171,p. 124 - 132]