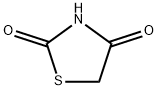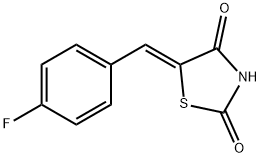
(Z)-5-(4-Fluorobenzylidene)thiazolidine-2,4-dione synthesis
- Product Name:(Z)-5-(4-Fluorobenzylidene)thiazolidine-2,4-dione
- CAS Number:291536-35-1
- Molecular formula:C10H6FNO2S
- Molecular Weight:223.22
Yield:291536-35-1 97%
Reaction Conditions:
with Fe3O4/SiO2-NH2/Cu(II) in ethanol; for 0.5 h;Catalytic behavior;Reflux;Green chemistry;
Steps:
General procedure: To a mixture of thiazolidine-2,4-dione or rhodanine(0.13 g, 1 mmol), aliphatic/aromatic aldehyde (1 mmol), and ethanol (4 ml), a portion of Fe3O4/SiO2-NH2/Cu(II) (0.15 g) was added and the mixture stirred under reflux for the required time (Table 2). The progress of the reaction was monitored by TLC (n-hexane/EtOAc, 2:1). After completion of the reaction, EtOH (3 ml) was added and the nanocatalyst was separated by an external magnet. The crude product was recrystallized from EtOH.
References:
Akhavan, Malihe;Foroughifar, Naser;Pasdar, Hoda;Khajeh-Amiri, Alireza;Bekhradnia, Ahmadreza [Transition Metal Chemistry,2017,vol. 42,# 6,p. 543 - 552]

459-57-4
595 suppliers
$6.00/25g

291536-35-1
9 suppliers
inquiry