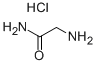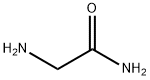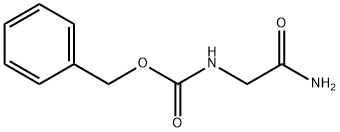
Z-GLY-NH2 synthesis
- Product Name:Z-GLY-NH2
- CAS Number:949-90-6
- Molecular formula:C10H12N2O3
- Molecular Weight:208.21
Yield: 86%
Reaction Conditions:
with sodium hydrogencarbonate;sodium carbonate in water;acetone at 20; for 3.5 h;
Steps:
2.3 2.2.3
Synthesis of Cbz-GlyNH2
The synthesis of Cbz-GlyNH2 was adapted from a previously reported protocol
[19]
.
Glycinamide hydrochloride (18 mmol, 2.00 g) was dissolved in water (60 mL) and acetone (8 mL), prior to the addition of Na2CO3 (54 mmol, 5.7 g) and NaHCO3 (18 mmol, 1.5 g).
Benzyl chloroformate (22 mmol, 3.20 mL) was added dropwise to the stirring solution over the course of 30 min.
The resulting mixture was stirred for 3 h at room temperature, after which the products were isolated by washing with diethyl ether (50 mL).
The protected product was precipitated out of solution by the slow addition of 0.1 M HCl.
The precipitate was filtered and subsequently dried in vacuo to afford a white solid in 86% yield (3.24 g).
1H NMR (CDCl3, 400 MHz): δ 3.87 (s, 2H), 5.07 (s, 2H), 7.26 (m, 5H), 7.35 (s, 2H), 7.95 (s, 1H).
13C NMR (CDCl3, 100 MHz): δ 45.1, 67.3, 126.2, 126.9, 128.9, 136.3, 156.8, 170.1. HRMS (ESI) calculated for C10H12N2O3: 208.0848. Found: 208.0851.
References:
St-Jacques, Antony D.;Rachel, Natalie M.;Curry, Dan R.;Gillet, Steve M.F.G.;Clouthier, Christopher M.;Keillor, Jeffrey W.;Pelletier, Joelle N.;Chica, Roberto A. [Journal of Molecular Catalysis B: Enzymatic,2016,vol. 123,p. 53 - 61]
1138-80-3
450 suppliers
$6.00/10g

949-90-6
114 suppliers
$9.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

949-90-6
114 suppliers
$9.00/5g
55378-79-5
0 suppliers
inquiry

949-90-6
114 suppliers
$9.00/5g