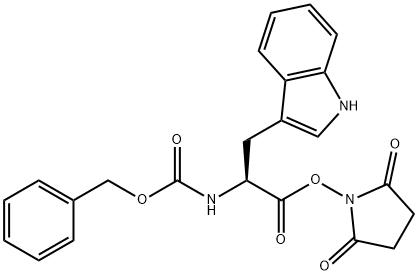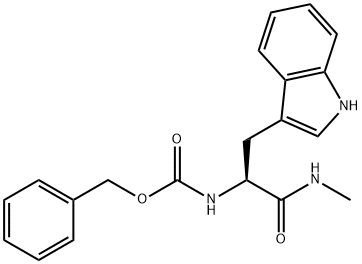
Z-TRP-NHME synthesis
- Product Name:Z-TRP-NHME
- CAS Number:53708-61-5
- Molecular formula:C20H21N3O3
- Molecular Weight:351.4
Yield:53708-61-5 100%
Reaction Conditions:
in tetrahydrofuran at 20; for 24 h;Inert atmosphere;
Steps:
7.1
Step 1 (S)-benzyl 3-(1H-indol-3-yl)-1-(methylamino)-1-oxopropan-2-yl carbamate (S)-2,5-dioxopyrrolidin-1-yl 2-(benzyloxycarbonylamino)-3-(1H-indol-3-yl)propanoate (1.5 g, 3.4 mmol) was dissolved in tetrahydrofuran (10 ml) in argon. Methylamine (2M in THF, 3.4 ml, 6.88 mmol) was added to this clear solution. A white precipitate separated out immediately after the addition. The reaction mixture was stirred for 24 h at room temperature. For work up the batch was adjusted to pH I with 2N HCl. The aqueous mixture was extracted with ethyl acetate (3*40 ml). The combined organic phases were washed with saturated sodium hydrogencarbonate solution (1*40 ml) and after drying with Na2SO4 were concentrated to low volume. The raw product was further processed in the next reaction without any further purification. Yield: 1.19 g (100%)
References:
US2009/247591,2009,A1 Location in patent:Page/Page column 21