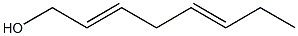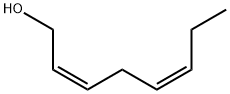
ZZ- 2,5-octadien-1-ol synthesis
- Product Name:ZZ- 2,5-octadien-1-ol
- CAS Number:67548-44-1
- Molecular formula:C8H14O
- Molecular Weight:126.2
Yield:67548-44-1 86 %Chromat. ,67548-36-1 6.3 %Chromat.
Reaction Conditions:
with quinoline;5% palladium on barium sulphate;hydrogen in methanol at 0 - 5; for 3.5 h;Temperature;
Steps:
(2Z,5Z)-2,5-Octadien-1-ol (23). 4.3.1. By catalytic hydrogenation.
5% Pd/BaSO4 (TCI, 1.15 g) was added to a solution of 22 (3.70 g, 30 mmol) and quinoline (1.0 mL)in MeOH (50 mL). The mixture was stirred vigorously at 0-5 C (ice-water) under H2 atmosphere (balloon). The H2 uptake ceased after 3.5 h. The mixture was filtered through Celite, and the Celite layer was washed with Et2O. The filtrate and washings were concentratedin vacuo, and the residue was dissolved in Et2O. The Et2O solution was successively washed with dil HCl, NaHCO3 solution and brine, dried (MgSO4), and concentrated in vacuo to give 3.34 g(87%) of 23. Distillation of a portion (2.32 g) of it gave 1.62 g of purified 23.
References:
Mori, Kenji [Tetrahedron,2015,vol. 71,# 34,p. 5589 - 5596]
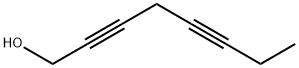
35378-76-8
9 suppliers
$120.00/10mg

67548-44-1
0 suppliers
inquiry
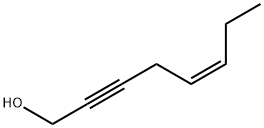
117606-34-5
0 suppliers
inquiry

67548-44-1
0 suppliers
inquiry

16400-32-1
138 suppliers
$12.00/250mg

67548-44-1
0 suppliers
inquiry

7348-78-9
8 suppliers
inquiry

67548-44-1
0 suppliers
inquiry