| Identification | More | [Name]
Bethanechol | [CAS]
590-63-6 | [Synonyms]
BETHANECHOL CHLORIDE
CARBAMYL-BETA-METHYLCHOLINE CHLORIDE
(2-hydroxypropyl)trimethylammoniumchloridecarbamate
2-((aminocarbonyl)oxy)-n,n,n-trimethyl-1-propanaminiuchloride
2-[(aminocarbonyl)oxy]-n,n,n-trimethyl-1-propanaminiuchloride
ammonium,(2-hydroxypropyl)trimethyl-,chloride,carbamate
beta-methylcholinechlorideurethan
bethainecholinechloride
carbaminoyl,beta-methylcholinechloride
carbamyl-b-methylcholinechloride*crystalline
carbamylmethylcholinechloride
duvoid
mechothane
myocholine
urecholine
urecholinechloride
Bethanechol chloride USP
BETHANECHOL CHLORIDE, PHARMA
(2-Hydroxypropyl)trimethylammonium chloride carbamate (6CI)
1-Propanaminium, 2-[(aminocarbonyl)oxy]-N,N,N-trimethyl-, chloride | [EINECS(EC#)]
209-686-8 | [Molecular Formula]
C7H17ClN2O2 | [MDL Number]
MFCD00055224 | [Molecular Weight]
196.68 | [MOL File]
590-63-6.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
BR5425000
| [TSCA ]
Yes | [HS Code ]
2924190002 |
| Hazard Information | Back Directory | [Hazard]
Headache, flushing, gastrointestinal distress, diarrhea, hypotension, excessive salivation, sweating, hypersensitivity. | [Description]
Bethanechol is an agonist of muscarinic acetylcholine receptors with IC50 values of 1,837, 25, 631, 317, and 393 μM for M1-5, respectively, in a radioligand binding assay using CHO cells expressing the human receptors.1 It inhibits M2-mediated increases in cyclic AMP induced by isoproterenol (Item No. 15592) in isolated guinea pig small intestine (IC50 = 127 μM).2 Bethanechol increases basal tone of isolated porcine intravesical ureter (EC50 = 4.27 μM).3 It also induces fluid secretion in the ileum, duodenum, and jejunum of anesthetized rats when administered at a dose of 60 μg/kg.4 Formulations containing bethanechol have been used to increase urination and improve smooth muscle tone in the gastrointestinal tract. | [Chemical Properties]
White Solid | [Originator]
Urecholine CI,MSD,US,1949 | [Uses]
A selective muscarinic receptor stimulant, used to treat cerebral palsy. | [Uses]
cholinergic | [Uses]
Therapeutic action of Betanechol is based on this action, and it is used for treating post�operational non-obstructive retention of urine and neurogenic bladder atony. Earlier, it was
used for treating gastrointestinal illnesses and Alzheimer’s disease. | [Definition]
ChEBI: The chloride salt of bethanechol. A slowly hydrolysed muscarinic agonist with no nicotinic effects, it is used to increase smooth muscle tone, as in the gastrointestinal tract following abdominal surgery, treatment of gastro-oesophageal reflux disease, and
as an alternative to catheterisation in the treatment of non-obstructive urinary retention. | [Manufacturing Process]
About 3 grams of β-methylcholine chloride are stirred at room temperature
with an excess of phosgene dissolved in 50 grams of chloroform, for about 2
hours. Excess phosgene and hydrochloric acid are removed by distillation
under vacuo. Additional chloroform is added to the syrup and the mixture is
poured into excess ammonia dissolved in chloroform and cooled in solid
carbon dioxide-acetone. The solid is filtered and extracted with hot absolute
alcohol. The solid in the alcohol is precipitated with ether, filtered, and
recrystallized from isopropanol. The carbaminoyl-β-methylcholine chloride
obtained has a melting point of about 220°C. | [Brand name]
Duvoid (WellSpring); Myotonachol (Glenwood);
Urecholine (Odyssey). | [Therapeutic Function]
Cholinergic | [General Description]
Bethanechol, β-methylcholinechloride carbamate, (2-hydroxypropyl)trimethylammoniumchloride carbamate, carbamylmethylcholinechloride (Urecholine), is nonspecific in its action on muscarinicreceptor subtypes but appears to be more effective ateliciting pharmacological action of M3 receptors. It haspharmacological properties similar to those of methacholine.Both are esters of β-methylcholine and have feeblenicotinic activity. Bethanechol is inactivated more slowlyby AChE in vivo than is methacholine. It is a carbamyl esterand is expected to have stability in aqueous solutions similarto that of carbachol. | [Mechanism of action]
Bentanechol is a drug, which has structurally unique qualities of both methacholine and
carbachol, i.e. it contains both β-methyl and carbamate functional groups, and quite
logically exhibits pharmacological properties of both the drugs. It is resistant to hydrol�ysis by cholinesterases and has a very minor effect on nicotinic receptors of the
autonomic ganglia and neuromuscular junctions. Betanechol has more of a selective
action on muscarinic receptors of the gastrointestinal tract and the bladder than do other
cholinic esters. | [Clinical Use]
The main use of bethanechol chloride is in the relief ofurinary retention and abdominal distention after surgery.The drug is used orally and by subcutaneous injection. Itmust never be administered by intramuscular or intravenousinjection because of the danger from cholinergic overstimulationand loss of selective action. Proper administration ofthe drug is associated with low toxicity and no serious sideeffects. Bethanechol chloride should be used with caution inasthmatic patients; when used for glaucoma, it producesfrontal headaches from the constriction of the sphinctermuscle in the eye and from ciliary muscle spasms. Its durationof action is 1 hour. | [Synthesis]
Betanechol, 2-carbamoyloxy-1-(N,N,N-trimethyl)propyl ammonium chlo�ride (13.1.8), is made by either the subsequent reaction of 1-(N,N,N-trimethylammonium)
propan-2-ol with phosgene, followed by ammonia, or by a completely analogous
synthesis of carbachol by the reaction of 1-chloro-2-propanol with phosgene followed by
consequent reactions with ammonia, and then with trimethylamine, giving betanechol
(13.1.8) [14,15].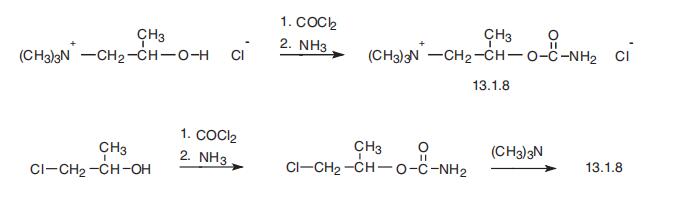
| [Veterinary Drugs and Treatments]
In veterinary medicine, bethanechol is used primarily to stimulate
bladder contractions
in small animals. It also can be used as an
esophageal or general GI stimulant, although metoclopramide
and/
or neostigmine have largely supplanted it for these uses. |
|
|