| Company Name: |
HUNAN CHEMFISH SCIENTIFIC CO.,LTD
|
| Tel: |
13021512891 |
| Email: |
sales04@chemfish.com |
| Products Intro: |
Product Name:chlorine trifluoride
CAS:7990-91-2
Package:1kg
|
|
| | Chlorine trifluoride Basic information |
| | Chlorine trifluoride Chemical Properties |
| RIDADR | UN 1749 2.3 | | HazardClass | 2.3 |
| | Chlorine trifluoride Usage And Synthesis |
| Description | Chlorine trifluoride (CIF3) is a toxic, corrosive, very reactive liquefied compressed gas packaged in cylinders as a liquid under its own vapor pressure of 1.55 kg/cm2 at 21°C (22 psia at 70°F). CIF3 is a very useful chemical in operations requiring a highenergy fluorinating agent or incendiary material, especially since it can be handled at room temperatures.
Chlorine trifluoride is primarily of interest as a component in rocket fuels, in industrial cleaning and etching operations primarily in the semiconductor industry, nuclear reactor fuel processing and other industrial operations. | | Chemical Properties | Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. Boils at 53°F. It reacts with water to form chlorine and hydrofluoric acid with release of heat.
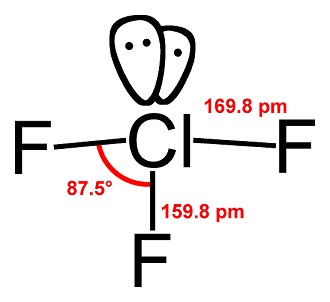 | | History | Fluorine (F2) has been recognized as the most powerful oxidizing agent of all known elements. Due to difficulties handling F2 in its most reactive state (liquid), substitutes were evaluated in the late 1920s to find similarly reactive compounds with easier handling. Ruff and Krug successfully isolated Chlorine trifluoride in 1930 after experimental tests with chlorine monofluoride suggested the presence of a higher fluoride species.Liquid Chlorine trifluoride is considered more reactive than vapor-phase F2 reactions since more moles of fluorinating agent are present per unit area of reactant surfaces. Also, liquid Chlorine trifluoride may demonstrate even higher reactivity in certain circumstances than liquid F2 because the F2 liquid temperature is cryogenic, thus reducing its activity potential.
German interest in Chlorine trifluoride during World War II prompted the first industrial bulk production capability for the material. The Germans produced CIF3 in tonnage quantities for military use in flamethrowers due the liquid’s extreme hypergolic nature with fuels (self-igniting) and as a general incendiary material. Following the war, interest in the use of CIF3 for organic synthesis work increased although the material was eventually considered to be too reactive for practical use and mostly abandoned for these applications.Synthesis reactions proved difficult to control and usually led to a wide variety of reaction by-products that were hazardous.
Many fluorinating compounds were evaluated as potent oxidizers for liquid–fueled rockets in the late 1940s through the early 1950s to overcome the storage and handling disadvantages of liquid F2. Chlorine trifluoride was first tested in the U.S. in 1948 on a liquid propellant rocket motor using hydrazine as the fuel. Additional testing yielded favorable results. However, all rocket materials of construction (including metals and seals) that could contact CIF3 had to be scrupulously selected, cleaned, and passivated to prevent the components from burning during reaction.CIF3 was also recognized as an extremely hazardous propellant due to its reactivity, toxicity, and toxic by-products of fluorination. | | Uses | Chlorine trifluoride is used as a fluorinating agent. It may be Used as a fluorinating agent, incendiary, igniter and propellant for rockets, in nuclear reactor fuel processing, pyrolysis inhibitor for fluoro carbon polymers.
During Word War II, Chlorine trifluoride was used by Germany as an incendiary gas. | | Preparation | Chlorine trifluoride was first reported by Ruff and Krug who prepared it by fluorination of chlorine, this also produced ClF and the mixture was separated by distillation.
3F2 + Cl2 → 2ClF3 | | Health Hazard | Chlorine trifluoride is toxic by itself and also reacts with moisture to form a variety of other toxic and corrosive materials, including hydrofluoric acid. When the product escapes into the environment, it hydrolyzes with the moisture in the air or, in the case of human contact, with the moisture in the human body. Direct contact with CIF3 vapor or liquid can result in a thermal burn in addition to the chemical burns produced by the hydrolysis products. | | Chemical Reactivity | Chlorine trifluoride is hypergolic (will initiate the combustion of many materials without an ignition source) with many materials. It is extremely reactive with most inorganic and organic materials. These reactions can be very violent or in some cases explosive. Therefore, all materials that come into contact with chlorine trifluoride must be evaluated.
Chlorine trifluoride hydrolyzes rapidly with moisture to form mostly hydrogen fluoride along with hydrogen chloride, chlorine monofluoride, and a variety of oxyhalogen compounds. The oxyhalogens may include chlorine dioxide, chlorous acid, chlorine oxyfluoride and oxygen difluoride.
Chlorine trifluoride is a strong oxidizer that can essentially decrease the ignition temperature of potential fuels, including materials of construction (e.g., metals) for CIF3 systems. Furthermore, because of chlorine trifluoride’s extreme reactivity, there is a high potential for contamination to serve as an ignition source. Friction between two materials can generate fine particles (contaminants), which may ignite from the heat generated. Contaminants in chlorine trifluoride systems potentially can burn with sufficient heat to propagate the ignition to system components. | | Waste Disposal | Return unused product to the supplier for proper disposal. In process applications, gaseous chlorine trifluoride can be disposed of in either liquid or dry scrubbers. Dry scrubbers work well under normal operating conditions for small quantities of Chlorine trifluoride but are not recommended for large or emergency releases unless specifically designed. Scrubbers must be designed to withstand the heat generated in the event of a large release. For normal operation, it is recommended an inert gas be used as a diluents prior to product being introduced to scrubber. This will help disperse the heat of reaction. Wet scrubbers typically use caustic solutions, such as potassium or sodium hydroxide, as the scrubbing medium. Wet scrubbers handled the heat of reaction better, as well as neutralize the products of reaction. Disposal of liquid chlorine trifluoride is extremely hazardous and is not recommended. |
| | Chlorine trifluoride Preparation Products And Raw materials |
|