| Identification | More | [Name]
Sodium cyanide | [CAS]
143-33-9 | [Synonyms]
caswellno758
Cianuro di sodio
cianurodisodio
Cyanide of sodium
cyanideofsodium
Cyanobrik
Cyanogran
Cyanure de sodium
cyanuredesodium
cyanuredesodium(french)
Cymag
epapesticidechemicalcode074002
Hydrocyanic acid, sodium salt
hydrocyanicacid,sodiumsalt
Kyanid sodny
kyanidsodny
kyanidsodny(czech)
m-44cyanidecapsules
NaCN
prussiateofsoda | [EINECS(EC#)]
205-599-4 | [Molecular Formula]
CNNa | [MDL Number]
MFCD00003523 | [Molecular Weight]
49.01 | [MOL File]
143-33-9.mol |
| Chemical Properties | Back Directory | [Appearance]
Sodium cyanide is found as white granules, flakes or lumps. Sodium cyanide is shipped as pellets or briquettes. Odorless when dry. It absorbs water from air (is hygroscopic or deliquescent). Hydrogen cyanide gas released by sodium cyanide has a distinctive mild, bitter almond odor, but a large proportion of people cannot detect it; the odor does not provide adequate warning of hazardous concentrations. | [Melting point ]
563.7 °C(lit.) | [Boiling point ]
1497°C | [density ]
1.6 | [vapor density ]
1.7 (vs air)
| [vapor pressure ]
1 mm Hg ( 817 °C)
| [Fp ]
1500°C | [storage temp. ]
Poison room | [solubility ]
H2O: 1 M at 20 °C, clear, colorless
| [form ]
Solid | [pka]
9.36[at 20 ℃] | [color ]
White | [Odor]
The dry salts are odorless, but reaction with atmospheric moisture produces HCN,
whose bitter almond odor is detectable at 1 to 5 ppm; however, 20 to 60% of the
population are reported to be unable to detect the odor of HCN. | [PH]
11.7 (100g/l, H2O, 20°C) | [PH Range]
11-12 | [Water Solubility ]
37 g/100mL (20 ºC) | [Sensitive ]
Hygroscopic | [Merck ]
14,8605 | [BRN ]
3587243 | [Dielectric constant]
7.6(Ambient) | [Exposure limits]
TLV-TWA (measured as CN) skin 5 mg CN/m3 (ACGIH and OSHA), 5 mg CN/m3/ 10-minute ceiling (NIOSH). | [Stability:]
hygroscopic | [LogP]
-0.25 at 20℃ | [CAS DataBase Reference]
143-33-9(CAS DataBase Reference) | [NIST Chemistry Reference]
Sodium cyanide(143-33-9) | [EPA Substance Registry System]
143-33-9(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T+,N | [Risk Statements ]
R26/27/28:Very Toxic by inhalation, in contact with skin and if swallowed .
R32:Contact with acids liberates very toxic gas.
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S7:Keep container tightly closed .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S29:Do not empty into drains .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 1689 6.1/PG 1
| [WGK Germany ]
3
| [RTECS ]
VZ7525000
| [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
I | [HS Code ]
28371110 | [Safety Profile]
A deadly human poison by ingestion. A deadly experimental poison by ingestion, intraperitoneal, subcutaneous, intravenous, parenteral, intramuscular, and ocular routes. An experimental teratogen. Human systemic effects by ingestion: hallucinations, dstorted perceptions, muscle weakness, and gastritis. Experimental reproductive effects.
hydrocyanic acid physiologically, inhibiting tissue oxidation and causing death through asphyxia. Cyanogen is probably as toxic as hydrocyanic acid; the nitriles are generally considered somewhat less toxic, probably because of their lower volathty. The nonvolaule cyanide salts appear to be relatively nonhazardous systemically, so long as they are not ingested and care is taken to prevent the formation of hydrocyanic acid. Workers, such as electroplaters and picklers, who are daily exposed to cyanide solutions may develop a “cyanide” rash, characterized by itching and by macular, papular, and vesicular eruptions. Frequently there is secondary infection. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
moisture, acid. Many cyanides evolve hydrocyanic acid rather easily. This is a flammable gas and is highly toxic. Carbon dioxide from the air is sufficiently acidc to liberate hydrocyanic acid from cyanide solutions. Explodes if melted with nitrite or chlorate @ about 450”. Violent reaction with F2, Mg, nitrates, HNO3, nitrites. Upon contact with acid, acid fumes, water, or steam, it wdl produce toxic and flammable vapors of CNand NanO. Used in the extraction of gold and silver ores, in electroplating, and in insecticides. See also CYANIDE and HYDROCYANIC ACID,
The volaule cyanides resemble
Flammable by chemical reaction with heat, | [Hazardous Substances Data]
143-33-9(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
A clear colorless aqueous solution. | [Reactivity Profile]
SODIUM CYANIDE SOLUTION(143-33-9) is weakly basic. Reacts with acids of all kinds to generate quantities of very poisonous hydrogen cyanide gas. Incompatible with strong oxidizing agents, especially if solution dries out. Gives insoluble products with silver(I), mercury(I) and lead(II) ions that may decompose violently under certain conditions. | [Air & Water Reactions]
Slowly evolves flammable and poisonous hydrogen cyanide gas. | [Hazard]
Toxic by ingestion and inhalation. | [Health Hazard]
Sodium cyanide is a white crystalline solid that is odorless when dry, but emits a slight
odor of hydrogen cyanide in damp air. It is slightly soluble in ethanol and formamide. It is very poisonous. It explodes if melted with nitrite or chlorate at about 450°F. It produces
a violent reaction with magnesium, nitrites, nitrates, and nitric acid. On contact with acid,
acid fumes, water, or steam, it produces toxic and flammable vapors. Synonyms for sodium
cyanide are hydrocyanic acid, sodium salt, and cyanide of sodium. | [Potential Exposure]
Sodium cyanide is used as a solid or in solution to extract metal ores, in electroplating and metal cleaning baths; in metal hardening; in treatment of rabbit and rat burrows and holes and termite nests; in insecticides | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Use amyl nitrate capsules if symptoms develop. All area employees should be trained regularly in emergency measures for cyanide poisoning and in CPR. A cyanide antidote kit should be kept in the immediate work area and must be rapidly available. Kit ingredients should be replaced every 1�2 years to ensure freshness. Persons trained in the use of this kit; oxygen use, and CPR must be quickly available. | [Shipping]
UN1689 Sodium cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
Sodium cyanide decomposes on contact with acids, acid salts, water, moisture, alcohols, and carbon dioxide, releasing highly toxic and flammable hydrogen cyanide gas. Aqueous solution is a strong base; it reacts violently with acid and is corrosive. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Absorbs moisture from the air forming a corrosive syrup. Corrosive to active metals, such as aluminum, copper, and zinc. Under acid conditions, sarin hydrolyzes to form hydrofluoric acid. | [Description]
Sodium cyanide, NaCN, is a cyanide salt that is a white, deliquescent, crystalline powder and is soluble in water. The specific gravity is 1.6, which is heavier than water. Sodium cyanide is toxic by inhalation and ingestion, with a TLV of 4.7 ppm and 5 mg/m3 of air. The target organs are the cardiovascular system, central nervous system, kidneys, liver, and skin. Reactions with acids can release flammable and toxic hydrogen cyanide gas. Cyanides are incompatible with all acids. The four-digit UN identification number is 1689.
The NFPA 704 designation is health 3, flammability 0, and reactivity 0. The primary uses are in gold and silver extraction from ores, electroplating, fumigation, and insecticides. | [Waste Disposal]
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Add strong alkaline hypochlorite and react for 24 hours. Then flush to sewer with large volumes of water. | [Physical properties]
Physical Properties White cubic crystals; hygroscopic; density 1.6 g/cm3; melts at 563°C; very soluble in water; aqueous solution strongly alkaline and decomposes rapidly. | [Definition]
sodium cyanide: A white orcolourless crystalline solid, NaCN,deliquescent, soluble in water and inliquid ammonia, and slightly solublein ethanol; cubic; m.p. 564°C; b.p.1496°C. Sodium cyanide is now madeby absorbing hydrogen cyanide insodium hydroxide or sodium carbonatesolution. The compound is extremelypoisonous because it reacts with the iron in haemoglobin in theblood, so preventing oxygen reachingthe tissues of the body. It is used inthe extraction of precious metals andin electroplating industries. Aqueoussolutions are alkaline due to salt hydrolysis. | [Production Methods]
Sodium cyanide was first prepared in 1834 by heating
Prussian Blue, a mixture of cyanogen compounds of iron,
and sodium carbonate and extracting sodium cyanide from
the cooled mixture using alcohol. Sodium cyanide remained
a laboratory curiosity until 1887, when a process was patented
for the extraction of gold and silver ores by means of a dilute solution of cyanide. | [Reactions]
Sodium cyanide, NaCN, white solid, soluble, very poisonous, formed (1) by reaction of sodamide and carbon at high temperature, (2) by reaction of calcium cyanamide and sodium chloride at high temperature, reacts in dilute solution in air with gold or silver to form soluble sodium gold or silver cyanide, and used for this purpose in the cyanide process for recovery of gold. The percentage of available cyanide is greater than in potassium cyanide previously used. Used as a source of cyanide, and for hydrocyanic acid. | [Flammability and Explosibility]
Sodium cyanide and potassium cyanide are noncombustible solids. Reaction with
acids liberates flammable HCN. | [Industrial uses]
sodium
cyanide and other water-soluble cyanides are used as modifying reagents for selective
flotation of ores containing galena, sphalerite and gangue minerals. | [storage]
In
particular, work with cyanides should be conducted in a fume hood to prevent
exposure by inhalation, and splash goggles and impermeable gloves should be worn
at all times to prevent eye and skin contact. Cyanide salts should be stored in a cool,
dry location, separated from acids. |
| Questions And Answer | Back Directory | [Chemical Properties]
Sodium cyanide is a white crystalline solid that is odourless when dry but emits a slight odour of HCN in damp air. It is slightly soluble in ethanol and formamide. It is very poisonous. It explodes if melted with nitrite or chlorate at about 450°F. It produces a violent reaction with magnesium, nitrites, nitrates, and nitric acid. On contact with acid, acid fumes, water, or steam, it will produce toxic and flammable vapours.for the extraction of gold and silver ore, copper, zinc, carburizing, medicine and so on. For metallurgy, steel quenching, electroplating, extraction (forming cyanide), organic synthesis of raw materials, insecticidal and anti-corrosion.

| [Toxicity]
Sodium cyanide binds to the ferric iron of oxidized cytochrome oxidase, disabling its ability to deliver oxygen, resulting in tissue hypoxia, "intracellular asphyxia." Rat: oral administration-LD50: 6.44mg / kg, adult lethal dose 200mg. Being highly toxic.
It can be absorbed through the respiratory tract, digestive tract and skin. Animals, after inhaling sodium cyanide aerosol of 40mg-90mg / m3, get symptoms of irritations, irritability and salivation after 25 to 43 minutes. Inhalation of 150mg ~ 170mg / m3 for 62 to 76 minutes or inhalation of 400mg ~ 500mg / m3 for 20 minutes can cause death. Human oral LD50 is about 1mg ~ 2mg / kg. Under normal conditions of production, sodium cyanide dust is often inhaled at room temperature, and sodium vapor can be inhaled during heat treatment. Misdiagnosis is also one of the common causes of poisoning. In the event of a fire, try to prevent the generation of toxic hydrogen cyanide gas. Do not use carbon dioxide or acid-base foam fire extinguishers. Fire-fighting operation personnel must wear protective equipment, try not to contact with water containing sodium cyanide. This product is toxic with poisoning causing dizziness and other uncomfortable symptoms. When found, patients should immediately leave the contaminated area to the fresh air and taken 1% soda solution as first aid, at the same time, go to the hospital for treatment. UN No.: 1689/6257 / 6.1-04 / 215.
| [Uses]
For the extraction of gold and silver from ores; gold (or silver) reacts with sodium cyanide in the presence of air to form the complex sodium cyanurate, which dissolves the gold from the ore. Further reaction with zinc can displace gold, generating sodium cyanate and free the gold out.
4Au + 8NaCN + O2 + 2H2O → 4Na [Au (CN) 2] + 4NaOH
Na [Au (CN) 2] + Zn → Na [Zn (CN) 3] + Au
Others can also be used for iron blue (intermediate sodium ferrocyanide production), cyanuric chloride (intermediate product of cyanide production), plating bath (copper, cadmium and other plating, DL-methionine synthesis). It can act as liquid steel carburizing agent with barium chloride (usually accompanied with a bath temperature of 800 °C or more; should add salts that don’t cause NaCN evaporation at high temperature) , and for pesticides and other purposes.
| [Preparation]
1.(Castner improved method) sodium metal and ammonia as raw material to generate sodium amide, and then subjects to carbon reduction at 700 ~ 800 ℃to obtain the product [1].
2Na + 2NH3 → 2NaNH2 + H2
NaNH2 + C → [1] + H2
2. Natural gas (methane), ammonia, air as raw materials; their mixture is passed through the catalyst bed at 1000 ℃to generate hydrogen cyanide, followed by reaction with sodium hydroxide to obtain it [1].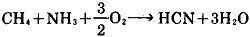
HCN + NaOH → [1] + H2O
3. Hydrogen cyanide can obtained as byproduct during ammonia oxidation of propylene to generate acrylonitrile [1].
|
|