| Identification | More | [Name]
2-Aminoadenosine | [CAS]
2096-10-8 | [Synonyms]
2,6-DIAMINOPURINE RIBOSIDE
2-AMINE ADENOSINE
2-AMINOADENOSINE
(2R,3R,4S,5R)-2-(2,6-Diaminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
2-AMINOADENOSINE HPLC98+% | [EINECS(EC#)]
606-677-4 | [Molecular Formula]
C10H14N6O4 | [MDL Number]
MFCD00053556 | [Molecular Weight]
282.26 | [MOL File]
2096-10-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White Powder | [Melting point ]
241-243°C (dec.) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
Aqueous Acid (Slightly, Heated, Sonicated), DMSO (Slightly, Sonicated), Ethanol | [Boiling point ]
798.5±70.0 °C(Predicted) | [density ]
2.25±0.1 g/cm3(Predicted) | [form ]
Powder | [pka]
13.10±0.70(Predicted) | [color ]
White to Off-White | [InChIKey]
ZDTFMPXQUSBYRL-UUOKFMHZSA-N | [CAS DataBase Reference]
2096-10-8(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
2-Aminoadenosine is a known analogue of adenosine that engages in three hydrogen bonds of the W-C type, adding considerable stability to the double helix. | [Chemical Properties]
White Powder | [Uses]
A nucleoside analog as inhibitor or substrate of adenosine kinase from M. tuberculosis. | [Definition]
ChEBI: 2-Aminoadenosine is a purine nucleoside. | [Synthesis]
Assays were performed in 5 and 10% of aprotic dipolar co-solvents, at 60°C, twelve units/ml cell lysate were added to 150 ml of a solution kept thermostatically at 60 °C, and having the following composition of substrate solutions:15mM Uridine/2 ' -Deoxyuridine,5 mM 2,6-Diaminopurine, and 30 mM potassium phosphate buffer, pH 7. After 5 hours, the reaction mixture was filtered by centrifugation at 2000 x g for 30 min, at 4°C, through an Amicon ultra-4 Centrifugal Filter Devices (Millipore, Bedford, MA) with a 3000-Da cutoff, and the filtrate was recovered. 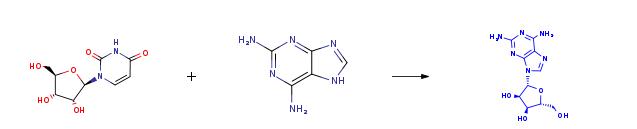 | [References]
Pyrazolo[3,4-d]pyrimidine ribonucleosides related to 2-aminoadenosine and isoguanosine: synthesis, deamination and tautomerism.
https://doi.org/10.1039/B708736E
An Efficient Process for Synthesis of 2′-O-methyl and 3′-O-methyl Guanosine from 2-aminoadenosine Using Diazomethane and the Catalyst Stannous Chloride.
http://dx.doi.org/10.1080/15257770500544529 |
|
|