| Identification | More | [Name]
Mepivacaine | [CAS]
22801-44-1 | [Synonyms]
MEPIVACAINE
N-(2,6-Dimethylphenyl)-1-Piperidinecarboxamide
N-(2,6-Dimethylphenyl)-1-methylpiperidine-2-carboxamide | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C15H22N2O | [MDL Number]
MFCD00243006 | [Molecular Weight]
246.35 | [MOL File]
22801-44-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
147-151°C | [storage temp. ]
-20°C Freezer | [solubility ]
Chloroform (Slightly), DMSO (Slightly), Ethanol (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
pKa 7.73(H2O,t =25±0.2,I=0.01(NaCl)) (Uncertain) | [color ]
White to Off-White | [InChI]
InChI=1S/C15H22N2O/c1-11-7-6-8-12(2)14(11)16-15(18)13-9-4-5-10-17(13)3/h6-8,13H,4-5,9-10H2,1-3H3,(H,16,18) | [InChIKey]
INWLQCZOYSRPNW-UHFFFAOYSA-N | [SMILES]
N1(C)CCCCC1C(NC1=C(C)C=CC=C1C)=O | [Uses]
Local anesthetic. | [CAS DataBase Reference]
22801-44-1(CAS DataBase Reference) | [EPA Substance Registry System]
22801-44-1(EPA Substance) |
| Hazard Information | Back Directory | [Originator]
Carboraine,Winthrop,US,1960 | [Definition]
ChEBI: Mepivacaine is a piperidinecarboxamide in which N-methylpipecolic acid and 2,6-dimethylaniline have combined to form the amide bond. It is used as a local amide-type anaesthetic. It has a role as a local anaesthetic and a drug allergen. | [Manufacturing Process]
Ethyl magnesium bromide is prepared in the usual way by reacting 185 parts by weight of ethyl bromide in 800 parts of anhydrous ether with 37 parts by weight of magnesium turnings. Under vigorous stirring 121 parts of 2,6- dimethyl aniline are added at a rate depending on the vigor of the gas evaporation. When the evolution of gas has ceased, 85 parts by weight of Nmethylpipecolic acid ethyl ester are added to the 2,6-dimethyl aniline magnesium bromide slurry. The mixture is refluxed for ? hour with continued stirring, after which it is cooled down. Dilute hydrochloric acid is added carefully in order to dissolve and hydrolyze the magnesium compound formed.
The pH is adjusted to 5.5 and the water phase separated and extracted with additional ether in order to remove the surplus dimethyl aniline. After addition of an excess of ammonia to the solution, the reaction product, Nmethylpipecolic acid 2,6-dimethyl anilide, is recovered by extraction with isoamyl alcohol. The isoamyl alcohol solution is evaporated to dryness, the product dissolved in dilute hydrochloric acid, treated with charcoal and reprecipitated with NaOH. N-methylpipecolic acid 2,6-dimethyl anilide is obtained in crystalline form. | [Therapeutic Function]
Local anesthetic | [General Description]
Mepivacaine hydrochloride is available in 1% to 3% solutionsand is indicated for infiltration anesthesia, dental procedures,peripheral nerve block, or epidural block. The onset of anesthesiais rapid, ranging from about 3 to 20 minutes for sensoryblock. Mepivacaine is rapidly metabolized in the liver with50% of the administered dose excreted into the bile asmetabolites. The metabolites are reabsorbed in the intestineand excreted in the kidney with only a small percentage foundin the feces. Less than 5% to 10% of the administered dose isfound unchanged in the urine. The primary metabolic productsare the N-demethylated metabolite and the 3 and 4 phenolicmetabolites excreted as their glucuronide conjugates. | [Clinical Use]
Mepivacaine hydrochloride [N-(2, 6-dimethylphenyl)-1-methyl 2-piperidinecarboxamide
monohydrochloride] is an amino amide-type local anesthetic agent widely used to provide
regional analgesia and anesthesia by local infiltration, peripheral nerve block, and epidural and
caudal blocks. The pharmacological and toxicological profile of mepivacaine is quite similar to
that of lidocaine, except that mepivacaine has a slightly longer duration of action and lacks the
vasodilator activity of lidocaine. For this reason, it serves as an alternate choice for lidocaine
when addition of epinephrine is not recommended in patients with hypertensive vascular disease. | [Synthesis]
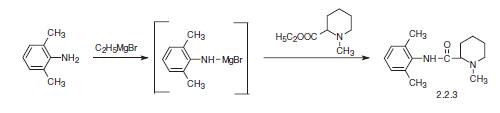
Mepivacaine is N-(2,6-dimethylphenyl)-1-methyl-2-piperindincarboxamide (2.2.3). Two primary methods of synthesis have been suggested. According to the first, mepivacaine is synthesized by reacting the ethyl ester of 1-methylpiperindine-2-carboxylic acid with 2,6-dimethylanilinomagnesium bromide, which is synthesized from 2,6- dimethylaniline and ethylmagnesium bromide [12–14].
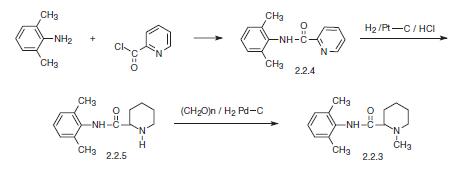
According to the figure below, reacting 2,6-dimethylaniline with the acid chloride of pyri�dine-carboxylic acid first gives the 2,6-xylidide of α-picolinic acid (2.2.4). Then the aro�matic pyridine ring is reduced to piperidine by hydrogen in the presence of a platinum on carbon catalyst.
The resulting 2,6-xylidide α-pipecolinic acid (2.2.5) is methylated to mepivacaine using formaldehyde with simultaneous reduction by hydrogen in the presence of platinum on carbon catalyst [15].
| [Metabolism]
Mepivacaine undergoes extensive hepatic metabolism catalyzed by CYP1A2, with only a small
percentage of the administered dosage (<10%) being excreted unchanged in the urine. The major
metabolic biotransformations of mepivacaine are N-dealkylation (to give the N-demethylated
compound 2′,6′-pipecoloxylidide) and aromatic hydroxylations. These metabolites are excreted as
their corresponding glucuronides. |
|
|