| Identification | More | [Name]
Resveratrol | [CAS]
501-36-0 | [Synonyms]
3,4',5'-TRIHYDROXY-TRANS-STILBENE
3,4',5-TRIHYDROXY-TRANS-STILBENE
5-[(1E)-2-(4-HYDROXYPHENYL)ETHENYL]-1,3,BENZENEDIOL
5-[2-(4-HYDROXY-PHENYL)-VINYL]-BENZENE-1,3-DIOL
(E)-5-[2-(4-HYDROXYPHENYL)ETHENYL]-1,3-BENZENEDIOL
RESVERATROL
RESVERATROLE
TRANS-1,2-(3,4',5-TRIHYDROXYDIPHENYL)ETHYLENE
TRANS-3,4',5-TRIHYDROXYSTILBENE
TRANS-3,4,5-TRIHYDROXYSTILBENE
TRANS-3,5,4'-STILBENETRIOL
TRANS-RESVERATROL
1,3-Benzenediol, 5-[(1E)-2-(4-hydroxyphenyl)ethenyl]-
RESVERATROL POWDER EXT. 8%
Resveratrol50%,90%,95%,98%
Resveratrol99%
TaxusChinensisiRehd
(E)-5-[2-(4-hydroxyphenyl )-ethenyl-1,3-dihydroxybenzene
(E)-5-[2-(4-hydroxyphenyl)etheyl]-1,3-benzenediol
3',4',5-Trihydroxystlbene | [EINECS(EC#)]
610-504-8 | [Molecular Formula]
C14H12O3 | [MDL Number]
MFCD00133799 | [Molecular Weight]
228.24 | [MOL File]
501-36-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White to Tan Powder | [Melting point ]
253-255°C | [Boiling point ]
449.1±14.0 °C(Predicted) | [density ]
1.359±0.06 g/cm3(Predicted) | [storage temp. ]
−20°C
| [solubility ]
Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 20 mg/ml). | [form ]
Powder | [pka]
9.22±0.10(Predicted) | [color ]
Off-white | [Water Solubility ]
Soluble in water (3 mg/100mL), ethanol (50 mg/mL), DMSO (16 mg/mL), DMF (~65 mg/mL), PBS (pH 7.2) (~100µg/mL), methanol, and acetone (50 mg/mL). | [Usage]
Minor constituent of wine, correlated with serum lipid reduction and inhibition of platelet aggregation. Resveratrol is a specific inhibitor of COX-1, and it also inhibits the hydroperoxidase activity of COX-1. It has been shown to inhibit events a | [Detection Methods]
HPLC,NMR | [Merck ]
8158 | [Stability:]
Stable for 2 years as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. | [InChI]
InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | [InChIKey]
LUKBXSAWLPMMSZ-OWOJBTEDSA-N | [SMILES]
C1(O)=CC(/C=C/C2=CC=C(O)C=C2)=CC(O)=C1 | [LogP]
3.139 (est) | [CAS DataBase Reference]
501-36-0(CAS DataBase Reference) | [EPA Substance Registry System]
1,3-Benzenediol, 5-[(1E)-2-(4-hydroxyphenyl)ethenyl]- (501-36-0) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R37/38:Irritating to respiratory system and skin .
R41:Risk of serious damage to eyes.
R36/38:Irritating to eyes and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S39:Wear eye/face protection .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [RTECS ]
CZ8987000
| [HS Code ]
29072900 | [Hazardous Substances Data]
501-36-0(Hazardous Substances Data) |
| Questions And Answer | Back Directory | [Resveratrol Benefits and ralting biological activities]
Much of the research pointing to the benefits have been laboratory or animal-based studies. So far, research on resveratrol's effectiveness in humans has yielded mixed results.
Anti-Aging and Anti-Cancer Effects
- Effects on biotransformation enzymes
- Inhibition of proliferation and induction of apoptosis
- Inhibition of tumor invasion and angiogenesis
Anti-inflammatory effects
- Protects Cardiovascular Health
- Inhibition of vascular cell adhesion molecule (VCAM) expression
- Inhibition of vascular smooth muscle cell (VSMC) proliferation
- Stimulation of endolethelial nitric oxide synthase (eNOS) activity
- Inhibition of platelet activiation and aggregation
Helps Protect the Brain and Cognitive/Mental Health
- Stimulation of neurogenesis and microvessel formation
- Stimulation of β-amyloid peptide clearance
- Inhibition of neuroinflammation
- Reduction of oxidative stress
| [resveratrol sources plants]
Resveratrol is anthraquinone terpenoids. It mainly comes from rhizome extract of polygonum cuspidatum. Polygonum cuspidatum: Shrubby perennial herb, up to 1 meter or more. Rhizome lying underground, wood brown, section significantly. Stems erect and cylindrical, surface glabrous, scattered with most red spots, hollow. Leaves alternate, broadly ovate to nearly circular, long 7-12cm, width 5-9cm, apex mucronate, base rounded or cuneate; petiole 1-1.5cm sheath care quality, brown, early fall. Flowering is from July to September, and fruiting from September to October. Spring and autumn can be excavated, cut and dried. Polygonum cuspidatum is born in the valley, creek or shore.
| [Resveratrol Extraction]
Polygonum cuspidatum is the rhizome of polygonaceae polygonum cuspidatum. Polygonum cuspidatum rhizome contains free anthraquinone and anthraquinone glycosides, whch mainly are emodin ether, rhubarb phenol, anthracene glycosides A and B, etc. It also contains resveratrol glycosides, polydatin, protocatechuic acid, dextrose catechin, tree anthrone-8-glucosidecassia, β-sitosterol glucoside and glucose, rhamnose, ginger candy, amino acids, tannin and copper, iron, manganese, zinc, potassium and potassium salts, etc.

Figure 1 is Polygonum cuspidatum plant
Resveratrol (Res) is a non-flavonoid polyphenol compound. It is considered as a phytoalexin, which is generated when the plant is attacked by pathogenic, or in the poor environment. It is mainly found in grapes, giant knotweed, peanuts, mulberry, pine, Gnetum, Korea Huaifrom 12 families and 31 genera of 72 species of plants.
Extraction of the natural plant: polygonum cuspidatum is used as raw material, extracted, extracted resveratrol crude, and then purified. Crude extraction technologies include organic solvent extraction method, the new method of alkaline extraction and enzymatic extraction method. New methods like microwave-assisted extraction, CO2 supercritical extraction and ultrasonic extraction are also applied. The main purpose of purification is to extract cis-resveratrol and trans-resveratrol and polydatin from raw resveratrol to obtain trans-resveratrol. Common methods of purification are chromatography, silica gel column chromatography, thin-layer chromatography, high performance liquid chromatography and the like.
Resveratrol had significant antioxidant activity on animal fats. Sample of 240mg/kg shows the strongest antioxidant activity during the three samples whose concentration of resveratrol is respectively 120 mg/kg, 240mg/kg, 360mg/kg. In antioxidant tests on lard, the antioxidant effect of resveratrol is stronger than polyphenols with same concentration.
| [Contents determination method]
C18 column, mobile phase consisted of acetonitrile and water (volume ratio 30: 70), and UV detection at 30 6nm are used to detect. The results show that if the concentration of resveratrol is in 10~250 μg/mL, concentration and peak area show a good linear relationship (r = 0.9999); the recovery is 925% to 1026%; the lowest detectable concentration is 0.6mg/g. The flow rate can be adjusted to detect the enzymatic conversion of polydatin and resveratrol at the same time. | [Pharmacological effects]
Resveratrol is not only the chemopreventive agents of neoplastic diseases, but also the chemopreventive agents to reduce platelet aggregation and prevent and treat atherosclerosis, cardiovascular and cerebrovascular disease. In the 1990s, studies of science and technology workers on resveratrol have advanced, and reveal its pharmacological effects. It can inhibit normal platelet aggregation, prevent myocardial cram, cerebral embolism. It has a protective effect on cardiac hypoxia, restore decline of cardiac output which is caused by burn or hemorrhagic shock, and can expand arteries and improve microcirculation.
In 1998, United States Al.Mindell listed resveratrol as one of “the 100 most popular and effective anti-aging substance” when he compiled the anti-aging Scripture. Peanut oil, peanut butter and other foods rich in resveratrol will be the new fashion of nutrition and health in the 21st century. Resveratrol is the chemopreventive agents of neoplastic diseases, but also the chemopreventive agents to reduce platelet aggregation and prevent and treat atherosclerosis, cardiovascular and cerebrovascular disease. Resveratrol shows some inhibitory effect on staphylococcus aureus, the card he bacteria, escherichia coli, pseudomonas aeruginosa, and has a stronger inhibitory effect on the orphan virus, herpes simplex virus, and enteroviruses, coxsackie a, b groups.
| [The synthetic method of resveratrol]
Resveratrol is an inhibitor of many oxidative metabolism enzymes of aromatic carcinogens. It is named as the natural chemopreventive agents of cardiovascular and cerebrovascular diseases and cancer. Many studies have shown that resveratrol also has many functions, such as anti-mutagenic activity, protecting cell toxicity induced by the oxidation of lipoprotein, inhibiting tumor cell reproduction, and so on.
Human separated resveratrol from the root of veratrum grandiflorum for the first time in 1940. Researchers currently has found resveratrol from at least 21 families and 31 genera of 72 species of plants, such as grape vine genus, vitis snake, legumes arachis, cassia, sophora, polygonum, and so on. Because of the low concentration of resveratrol in the plant and the high extraction cost, using chemical, biological, genetic engineering and other methods to obtain resveratrol has become an indispensable means for its development process. The roadmap to synthesis resveratrol is as follows:
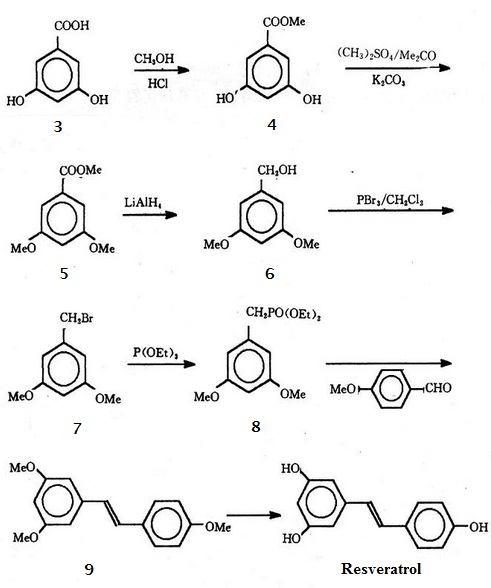
3,5-dihydroxybenzoic acid (3) forms 3,5-dihydroxybenzoic acid methyl ester (4) by esterification reaction. Choose methyl ether as phenolic hydroxyl-protecting groups. (4) reacts with dimethyl sulfate to get 3,5-di-methoxybenzoate (5). (5) generates the corresponding benzene methanol (6) after reduction, and forms 3, 5-dimethoxy benzyl bromide (7) through further bromination. Then diethyl (3,5-dimethoxy benzyl) phosphonate (8) can be get by roman abramovich's reaction. (8) reacts with p-anisaldehyde to form the key intermediate 3,4,5-trimethoxy-trans-stilbene (9) via Wittig-Honer reaction. Finally, methyl can be removed by freshly prepared aluminum triiodide. Then the aimed product resveratrol can be separated by thin layer chromatography. | [Solubility]
Resveratrol is a kinds of natural antioxidants that presents in grapes, red wine, mulberries, peanuts and polygonum cuspidatum. It is polyphenols. It is soluble in organic solvents but insoluble in water. Its dissolved order in organic solvents is: acetone > ethanol> methanol> ethyl acetate> ether> chloroform. | [Possible Side effects]
Little is known about the safety of long-term or high dose use of resveratrol.
Since resveratrol might act like estrogen, some medical experts recommend that people with hormone-sensitive cancers (condition such as breast cancer, uterine cancer, ovarian cancer, endometriosis, or uterine fibroids), pregnant women, and children avoid taking resveratrol.
In addition, resveratrol could interact with blood thinners like warfarin, aspirin, and ibuprofen by slow blood clotting, which may increase the risk of bleeding in people with bleeding disorders.
According to one study, high-dose resveratrol supplementation was associated with fever, reduced blood cells, and decreased blood pressure.
There is some concern that high doses of resveratrol supplements could lead to kidney problems in some people.
| [Uses]
- Resveratrol can prevent the oxidation of low density lipoprotein, and has the potential effect on preventing cardiovascular disease, cancer, antivirus and immune regulation. Its main role is antioxidant properties.
- Cardiovascular drugs. It can reduce hematic fat and prevent heart disease. It also has the effect on AIDS.
- Antioxidants and the activity in anti-inflammatory, antithrombotic, anti-cancer, anti-cancer, anti hyperlipidemia and antibacterial.
- Anti-aging, regulating blood lipid, cardiovascular protection, anti-hepatitis.
- Resveratrol is a phytoalexin produced naturally by several plants with anti-cancer, anti-inflammatory, blood-sugar-lowering and other beneficial cardiovascular effects.
|
| Hazard Information | Back Directory | [Chemical Properties]
Off-White to Tan Powder | [Definition]
ChEBI: A resveratrol in which the double bond has E configuration. | [Synthesis Reference(s)]
Tetrahedron Letters, 43, p. 597, 2002 DOI: 10.1016/S0040-4039(01)02227-4 | [Description]
Resveratrol, a non-flavonoid polyphenolic compound, is widely found in the skin of red grapes, nuts, berries, Polygonum cuspidatum root, etc. It is reportedly known to exhibit pharmacological properties including anti-cancer, anti-inflammatory, antioxidant, neuroprotectant, anti-atherogenic property and reduces the synthesis of pro-atherosclerotic substances. | [General Description]
Resveratrol, a non-flavonoid polyphenolic compound, is widely found in the skin of red grapes, nuts, berries, Polygonum cuspidatum root, etc. It is reportedly known to exhibit pharmacological properties including anti-cancer, anti-inflammatory, antioxidant, neuroprotectant, anti-atherogenic property and reduces the synthesis of pro-atherosclerotic substances. | [Biological Activity]
A phytoestrogen with antitumor, antioxidant, antiplatelet, anti-inflammatory and antifungal effects. Inhibits cytochrome P450 1A1 (IC 50 = 23 μ M) and displays mixed agonist/antagonist actions at ER α and ER β estrogen receptors. Converted into the anticancer agent piceatannol (4-[(1E)-2-(3,5-Dihydroxyphenyl)ethenyl]-1,2-benzenediol ) by cytochrome P450 1B1 | [Biochem/physiol Actions]
ED50 = 15 μM against COX-1 | [Mechanism of action]
Resveratrol can be found in the skins and seeds of grapes and in peanuts. It has demonstrated potent antioxidant, anti-inflammatory, and anti-proliferative activities. Topical application of resveratrol in mice demonstrated photoprotection by significantly decreasing UVB-mediated generation of hydrogen peroxide and infiltration of leukocytes. Its antiproliferative properties are related to the inhibition of cellular events associated with tumor initiation, promotion, and progression, and the triggering of apoptosis in tumor cells. | [Anticancer Research]
Resveratrol is a stilbinoid, found in the skin of grapes, peanuts, berries, and otherfruits. The cytotoxic effect of resveratrol is mediated via the inhibition of severaltranscription factors; upregulation of caspases, Bax, and p53; and downregulationof survivin, cyclins, and Bcl-2. Increase in Bax/Bcl-2 ratio and upregulation ofcaspases lead to apoptosis. The beneficial effects of resveratrol against cancer havebeen shown in all the stages of cancer including carcinogenesis, initiation,promotion, and progression. It could inhibit Wnt target gene expression in normalcolonic mucosa of the colorectal cancer patients. It increases the caspase-3 inmalignant hepatic tissue and induces anticarcinogenic effects in humangastrointestinal tract (Hosseini and Ghorbani 2015). It has the ability to inhibit thedevelopment of DMBA-induced phenoblastic lesions in the mammary gland organculture (MMOC) model of carcinogenesis and in two-stage full-term mouse model(Balunas and Kinghorn 2005). It prevents carcinogenesis by upregulating Bax andp53 proteins and downregulating NF-κB, COX-2, AP-1, cyclin-dependent kinases,hypoxia-induced factor 1α (HIF-1α), cyclins, MMPs, cytokines, and Bcl-2 proteins(Singh et al. 2016b). It plays a pivotal role in preventing the initiation, promotion,and progression of cancer by inducing phase II drug metabolizing enzymes, bymediating anti-inflammatory effects and inhibiting COX and hydroperoxidasefunctions, and by inducing cell differentiation, respectively, in human promyelocyticleukemic cells (Jang et al. 1997; Aggarwal et al. 2004).
Resveratrol, a noteworthy polyphenol occurring in different plants such as grapesand peanuts, has appeared to be required in cell reinforcement, anti-proliferative,anti-inflammatory, and chemopreventive activities. Various potential medicaladvantages, including decreased danger of malignancy and coronary illness, arebelieved to be related to the utilization of resveratrol. It can successfully and proficientlyrepress endothelial cell multiplication and migration, with little cell toxicityin the HUVEC and ARPE19 lines (Cao et al. 2010). Also, there have been perceptionsof inhibitory consequences for smooth muscle cell migration (Venkatesanet al. 2009) and tumor necrosis factor-alpha-incited monocyte adhesion and migration(Kim et al. 2007). As of late, it was recognized that the restraint of PDGF-BB-actuatedcell migration by resveratrol and the particular inhibitors PDGF-R, PI3K,MEK, or p38 in wound healing test agreed with diminished enactment of PDGFBB-incited PDGFR-β, PI3K/Akt, ERK, and p38 phosphory-lation in Western blotinvestigation, recommending that resveratrol hinders cell migration through theinhibition of PI3K/Akt, PDGFR-β, and MAPK cascade (Chan et al. 2013). | [storage]
+4°C | [References]
1) Fremont (2000), Biological effects of resveratrol ; Life Sci., 66 663
2) Howitz et al. (2003), Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan; Nature, 425 191
3) Park et al. (2012), Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases; Cell, 148 421 |
|
|