| Identification | More | [Name]
Thymine | [CAS]
65-71-4 | [Synonyms]
2,4-DIHYDROXY-5-METHYLPYRIMIDINE
2,4-DIHYDROXY-5-METHYLPYRIMIDINE 5-METHYLURACIL
5-METHYL-1,2,4-DIHYDROXYPYRIMIDINE
5-METHYL-2,4-DIHYDROXYPYRIMIDINE
5-METHYLPYRIMIDINE-2,4-DIOL
5-METHYLURACIL
D-THYMIDINE
LABOTEST-BB LT00848102
METHYL(5-) URACIL
THYMIN
THYMINE
2,4(1H,3H)-Pyrimidinedione, 5-methyl-
2,4(1H,3H)-Pyrimidinedione,5-methyl-
2,4-Dihydroxy-5-methylpyrimidin
3h)-pyrimidinedione,5-methyl-4(1h
4(1H,3H)-Pyrimidinedione,5-methyl-2
5-Methyl-2,4(1H,3H)-pyrimidinedione
5-Methyl-2,4-dioxypyrimidine
5-Methylpyrimidine-2,4-dione
thymin(purinebase) | [EINECS(EC#)]
200-616-1 | [Molecular Formula]
C5H6N2O2 | [MDL Number]
MFCD00006026 | [Molecular Weight]
126.11 | [MOL File]
65-71-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystalline powder | [Melting point ]
~320 °C (dec.) (lit.) | [Boiling point ]
234.21°C (rough estimate) | [density ]
1.3541 (rough estimate) | [refractive index ]
1.5090 (estimate) | [storage temp. ]
Store at RT. | [solubility ]
DMSO (Slightly), Methanol (Slightly, Heated) | [form ]
Powder | [pka]
9.94(at 25℃) | [color ]
White | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Water Solubility ]
Soluble in hot water. Slightly soluble in alcohol. | [Decomposition ]
335 °C | [Detection Methods]
HPLC,NMR | [Merck ]
14,9398 | [BRN ]
607626 | [InChIKey]
RWQNBRDOKXIBIV-UHFFFAOYSA-N | [LogP]
-0.620 | [CAS DataBase Reference]
65-71-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Thymine(65-71-4) | [EPA Substance Registry System]
65-71-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36:Irritating to the eyes.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
2
| [RTECS ]
XP2100000
| [TSCA ]
Yes | [HS Code ]
29335900 |
| Questions And Answer | Back Directory | [Thymine]
Thymine, also known as 5-methyl uracil, the earliest isolated from calf thymus and then got the name, and is the major pyrimidine component of deoxyribonucleic acid. And it can be linked with deoxyribose through the glycoside chain to form deoxythymidine, the triphosphate of deoxythymidine triphosphate, the triphosphoric acid of which can compound to deoxythymidine triphosphate, which is a precursor of thymine during deoxyribonucleic acid biosynthesis.
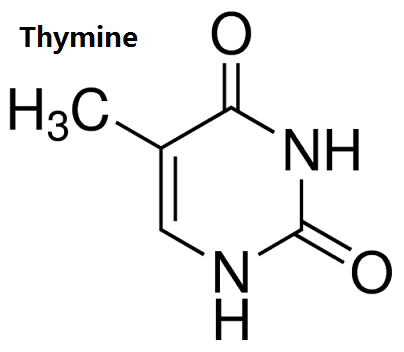
Figure 1 the structure of thymine. | [Physiological function]
Nucleotides consist of bases, pentoses and phosphates, and constitute the basic unit of biological macromolecular nucleic acid. According to the difference of pentose in nucleotide composition, nucleotides can be divided into ribonucleotides and deoxyribonucleotides, and there constitute the two types of nucleic acids: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). The Compositions of nucleotide base are two categories, pyrimidine and purine. Pyrimidine is a 6-membered heterocyclic organic compound containing two nitrogen atoms. Common pyrimidine bases in nucleotides are cytosine (C), uracil (V) and thymine (T). Purine is a heterocyclic compound with four-nitrogen. Adenine (A) and bird purine (G) are the major purine bases that make up the nucleotides.Purine and pyrimidine are heterocyclic nitrogen-containing compounds that are necessary for biological (including human) nucleic acid metabolism. Purine, pyrimidine and ribose and phosphate combine to form RNA; purine, pyrimidine and deoxyribose and phosphate combine to produce DNA.DNA is the main chemical constituent of genes, which plays an important role in the transmission of genes, and the main function of RNA is the regulation of intracellular protein synthesis. the final metabolism product of Purine is mainly uric acid. The clinically relevant purines are adenine and guanine. Important pyrimidines are thymine, cytosine and uracil. In the purine and pyrimidine metabolism must have all kinds of enzymes involved in to make the metabolic process according to normal procedure, the lack of any kind of these enzymes can cause the corresponding purine or pyrimidine metabolic disorders, resulting in clinical-specific symptoms such as whey aciduria Xanthineuria and Lesch-Nyhan syndrome. Others, such as primary children with gout; uraemia with partial phosphoribosyl transferase deficiency; female intelligence retardation, mutations and hyperuricemia syndrome; 2,8-dehydroadenurine and deaminase deficiency in children is rare.
This information was edited by Chemicalbook Xiao Nan (2015-08-03). | [The Four DNA Bases]
There are four bases that support the formation of DNA. They are thymine, adenine, guanine, and cytosine and are also known by the acronyms T, A, G, and C. These bases are attracted to one another and form specific partnerships to help in the creation of DNA. DNA is a small molecule found in every cell of your body and is responsible for writing your body's genetic information.
DNA is best visualized by picturing a long, twisted, spiraling ladder. The inner portion of the ladder is constructed of rungs. If you picture the four bases of DNA as rungs that assist with the formation of the ladder, you can get a solid understanding of how the bases hold the DNA structure together. Just as the rungs have a responsibility to stabilize the ladder, the bases have a responsibility to stabilize the structure of DNA.
Thymine is an interesting base because it is the only one of the four bases found exclusively inside of DNA. The other bases are also found in RNA, which is often thought of DNA's cousin because of the close relationship and joint assistance the two often share genetic information transfer process. | [Chemical properties]
White crystalline powder, insoluble in water at room temperature, slightly soluble in alcohol, soluble in alkali, acid, formamide, DMF and pyridine. Melting point is 320-330 ° C. | [Uses]
(1) Thymine, along with adenine, guanine, and cytosine, is one of the four nucleic acid bases in DNA; thymine can be used to study chemical processes that affect DNA structure, such as a radiation-induced free radical product resulting in base cross-linking reaction and derivatization reaction. Thymine can be used to study the energy and hydrogen bonding kinetic parameters with other nucleic acid bases such as adenine; thymine is used for the development of (Hg) detectors with sensitive, nanoparticle-based structures and coordination chemistry.
(2) 5-methyl pyrimidine is an important component of genetic material, is the key intermediates of synthestising anti-AIDS drugs AZT, DDT and related drugs key intermediates, but also the starting material of synthetising anti-tumor, antiviral drug β-thymidine. |
| Hazard Information | Back Directory | [Definition]
A nitrogenous
base found in DNA. It has a PYRIMIDINE
ring structure. | [Definition]
ChEBI: A pyrimidine nucleobase that is uracil in which the hydrogen at position 5 is replaced by a methyl group. | [Definition]
thymine: A pyrimidine derivativeand one of the major componentbases of nucleotides and the nucleicacid DNA. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 25, p. 149, 1960 DOI: 10.1021/jo01071a612 | [General Description]
Thymine (Zidovudine Related Compound C) is a process related impurity of antiretroviral drug zidovudine. Zidovudine belongs to the class of drugs known as nucleoside reverse transcriptase inhibitors and is generally used in the prevention and treatment of HIV/AIDS.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. | [Biochem/physiol Actions]
Thymine used in studying DNA structure byirradtaion methods leading to cross-linking reactions and derivitizations. Thymine dimers are indicative of DNA damage. Thymine is used with metal (mercury) to form thymine?HgII?thymine (T?HgII?T) duplexes. Thymine starvation in bacteria leads to halt in DNA synthesis and is referred as thymine-less death. | [Purification Methods]
Crystallise thymine from EtOAc, 10% aqueous EtOH or water. It has m 318-320o after sublimation at 200o/12mm. Purify it by preparative (2mm thick) TLC plates of silica gel, eluting with ethyl acetate/isopropanol/water (75:16:9, v/v; RF 0.75). The desired spot is localated with a uv lamp, cut the band from the plate, place it in MeOH, shake and filter it through a millipore filter, then evaporate. [Infante et al. J Chem Soc, Faraday Trans 1 68 1586 1973, Beilstein 24 H 353, 24 I 330, 24 II 183, 24 III/IV 1292.] |
|
|