| Identification | More | [Name]
Isoprene | [CAS]
78-79-5 | [Synonyms]
2-METHYL-1,3-BUTADIENE
2-METHYL-1,3-HEXADIENE
2-METHYL-BUTA-1,3-DIENE
ACETATE BUFFER
ACETATE BUFFER, PH 4.5
ACETATE BUFFER, PH 6.0
ACETATE BUFFER REAGENT
ACETATE BUFFER SOLUTION R-455
ACETATE BUFFER SOLUTION TYPE 'C'
ACETATE BUFFER TS
ACETIC ACID-SODIUM ACETATE
BUFFER ACETATE
BUFFER ACETATE, PH 4.00
BUFFER, PH 4.63
BUFFER PH 4.65
BUFFER PH7.20
BUFFER SOLUTION
BUFFER SOLUTION (ACETATE), PH 4.00
BUFFER SOLUTION (ACETATE), PH 4.0-4.5
BUFFER SOLUTION, PH 4.0, ACETATE | [EINECS(EC#)]
201-143-3 | [Molecular Formula]
C5H8 | [MDL Number]
MFCD00137248 | [Molecular Weight]
68.12 | [MOL File]
78-79-5.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless liquid with an aromatic odour | [Melting point ]
323-329 °C(lit.)
| [Boiling point ]
34 °C(lit.)
| [density ]
0.681 g/mL at 25 °C(lit.)
| [vapor density ]
2.35 (vs air)
| [vapor pressure ]
8.82 psi ( 20 °C)
| [refractive index ]
n20/D 1.422(lit.)
| [Fp ]
−65 °F
| [storage temp. ]
2-8°C
| [solubility ]
0.7g/l | [form ]
solid
| [pka]
>14 (Schwarzenbach et al., 1993) | [color ]
Clear colorless to very pale yellow | [Odor]
petroleum-like odor | [Stability:]
Stability Extremely flammable. Readily forms explosive mixtures with air. Note low flash point, low boiling point, high vapour pressure. Unstable-prone to spontaneous polymerization. May contain a polymerization inhibitor. Incompatible with strong oxidizing agents. | [explosive limit]
1-9.7%(V) | [Odor Threshold]
0.048ppm | [Water Solubility ]
0.07 g/100 mL | [FreezingPoint ]
-145.96℃ | [λmax]
231nm(neat)(lit.) | [Merck ]
14,5201 | [BRN ]
969158 | [Henry's Law Constant]
(x 10-2 atm?m3/mol):
3.45 at 18 °C (dynamic stripping cell-MS, Karl et al., 2003) | [Dielectric constant]
2.1(25℃) | [LogP]
2.42 at 20℃ | [CAS DataBase Reference]
78-79-5(CAS DataBase Reference) | [IARC]
2B (Vol. 60, 71) 1999 | [NIST Chemistry Reference]
1,3-Butadiene, 2-methyl-(78-79-5) | [EPA Substance Registry System]
78-79-5(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F+,T | [Risk Statements ]
R45:May cause cancer.
R12:Extremely Flammable.
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R68:Possible risk of irreversible effects. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 1218 3/PG 1
| [WGK Germany ]
1
| [RTECS ]
NT4037000
| [Autoignition Temperature]
428 °F | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
I | [HS Code ]
29012420 | [Hazardous Substances Data]
78-79-5(Hazardous Substances Data) | [Toxicity]
LD50 for mice: 144 mg isoprene vapors/l air (Gostinskii) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
3-Methyl-1-butene-->2-Methylbutane-->C5 Fraction | [Preparation Products]
Cyclopentene-->TERPINEOL-->3-Methyl-2-buten-1-ol-->2-Methyl-2-butene-->1-Chloro-3-methyl-2-butene-->2,2-DIMETHYLCYCLOPENTANONE-->6-Methyl-5-hepten-2-one-->butyl rubber-->METHYLIONONE-->3-METHYL-2,5-DIHYDROTHIOPHENE-1,1-DIOXIDE-->Methyl tetrahydrophthalic anhydride-->3-Methyl-2-butanone-->3,7-dimethyl-1,7-octadien-3-ol-->2,6-Dimethyl-1,7-octadien-3-ol-->3,6-dihydro-4-methyl-2-(2-methylpropyl)-2H-pyran-->DECANE,3,8-DIMETHYL- |
| Hazard Information | Back Directory | [General Description]
A clear colorless liquid with a petroleum-like odor. Density 5.7 lb/gal. Flash point-65°F. Boiling point 93°F. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a closed container, the container may rupture violently. Less dense than water and insoluble in water. Vapors heavier than air. | [Reactivity Profile]
ISOPRENE may react vigorously with strong oxidizing agents. May react exothemically with reducing agents to release hydrogen gas. May undergo exothermic addition polymerization in the presence of various catalysts (such as acids) or initiators. Undergoes autoxidation upon exposure to the air to form explosive peroxides. Mixing isoprene in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid (70%), oleum, sulfuric acid (90%) [NFPA 1991]. | [Air & Water Reactions]
Highly flammable. Insoluble in water. | [Hazard]
Highly flammable, dangerous fire and
explosion risk. Irritant. Possible carcinogen. | [Health Hazard]
Vapor produces no effects other than slight irritation of the eyes and upper respiratory tract. Liquid may irritate eyes; like gasoline. | [Physical properties]
Colorless, volatile, extremely flammable liquid with an petroleum-like odor. An odor threshold concentration of 48 ppbV was reported by Nagata and Takeuchi (1990). | [Uses]
Isoprene occurs in nature and it is produced by many plants. Its polymers are the main component of natural rubber. The most important application of isoprene is to manufacture polymers and copolymers. Polyisoprene, a synthetic rubber made from isoprene, is used in a wide variety of rubber applications including medical equipment, baby bottle teats/nipples, toys, shoe soles, tires, elastic films, threads for golf balls or textiles, adhesives, paints, and coatings. Copolymer butyl rubber, made from isobutene with a small amount of isoprene, has excellent impermeability to gases and is used in inner tubes. Another copolymer styrene-isoprene rubber is used in pressure sensitive adhesives. Isoprene is also used as a chemical intermediate.
| [Uses]
The majority of isoprene produced commercially is used to make synthetic rubber (cis-polyisoprene), most of which is used to produce vehicle tires. The second- and third-largest uses are in the production of styrene-isoprene-styrene block polymers and butyl rubber (isobutene-isoprene copolymer) (IARC 1994). | [Uses]
The primary use of isoprene is the manufacture of polyisoprene,
or ‘synthetic’ natural rubber, which is subsequently
used to make tires. Other major uses of isoprene include the
production of styrenic thermoplastic elastomer block copolymers
(styrene–isoprene–styrene) and butyl rubber (isobutene–
isoprene copolymer). Isoprene is also used to manufacture
other chemicals, intermediates, and derivatives, which are
subsequently used to manufacture vitamins, pharmaceuticals,
flavorings and fragrances, and epoxy hardeners. | [Definition]
ChEBI: A hemiterpene with the formula CH22C(CH3)CH2CH2; the monomer of natural rubber and a common structure motif to the isoprenoids, a large class of other naturally occurr
ng compounds. | [Preparation]
Isoprene is obtained from propylene by the followin,g route:
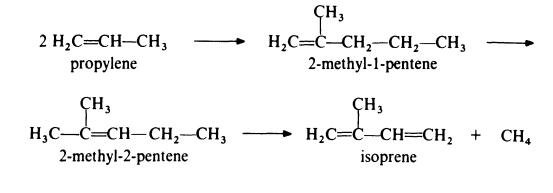
In the first step, propylene is dimerized to 2-methyl-l-pentene by passage
over a catalyst of tri-n-propylaluminium at about 200??C and 20 MPa (200
atmospheres). This product is then isomerized to 2-methyl-2-pentene by
heating at 150-300??C in the presence of a silica-alumina catalyst. The final
step in the process is the pyrolysis of the olefin to isoprene at 650-800??C in
the presence of a free radical initiator such as hydrogen bromide. The
isomerization step is necessary because pyrolysis of 2-methyl-l-pentene gives
much poorer yields of isoprene than pyrolysis of 2-methyl-2-pentene. | [Production Methods]
Rubber results from the polymerization of isoprene to form polyisoprene. The resultingstructure dictates the properties of the rubber. Natural rubber has a cis 1,4 structure.This means that the carbon atoms that form the chainattach to the same side ofthe chain at the 1 and 4 positions. The cisstructure gives rubber its elasticity. Polyisoprene alsoexists in a trans 1,3 configuration. In the trans configuration, the addition takes place onopposite sides of the carbon chain.
Natural rubber occurs in a colloidal milky suspension called latex, which is obtained fromnumerous plants. The most important of these is the para rubber tree, Hevea brasiliensis. Naturalrubber is harvested by cutting a v-shape incision into a plant and allowing latex to drain intoa container containing a preservative. About 50mL of latex is obtained on a daily basis. Latexis transported to collection stations where it is processed for shipment. Processing can includepreservation, coagulation, and concentrating before being sent to rubber factories. | [Flammability and Explosibility]
Extremelyflammable | [Carcinogenicity]
Isoprene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | [Environmental Fate]
At 25 ℃, isoprene has a high vapor pressure of 733 hPa, a low
water solubility of 642 mg l-1, and a Henry’s law constant of
7781 Pam3 mol-1. Isoprene’s log Kow is 2.42 while its log Koc
is 1.83. Isoprene’s vapor density relative to air is 2.4. Because of
its high vapor pressure at ambient temperature, isoprene will
partition largely into the atmosphere, with negligible amounts
partitioning to soil and water. Due to a short half-life in air
(0.5 h by reaction with nitric oxide, 1.2–4 h by reaction with
hydroxyl radicals, and 19 h by reaction with ozone), wet
deposition of isoprene from air is not expected to play
a significant role in its atmospheric fate. Although laboratory
testing demonstrates that isoprene has the potential to biodegrade,
microbial metabolism is unlikely to contribute significantly
to the removal of isoprene from the environment due to
rapid volatilization from terrestrial and aquatic media.
Isoprene has a low bioaccumulation potential and is not
expected to bioaccumulate. | [Purification Methods]
Reflux it with sodium then distil it from sodium or NaBH4 under nitrogen, and pass it through a column containing KOH, CaSO4 and silica gel. tert-Butylcatechol (0.02% w/w) is added, and the isoprene is stored in this way until redistilled before use. The inhibitor (tert-butylcatechol) in isoprene can be removed by several washings with dilute NaOH and water. The isoprene is then dried over CaH2, distilled under nitrogen at atmospheric pressure, and the fraction distilling at 32o is collected. Store it under nitrogen at -15o. [Beilstein 1 H 252, 1 IV 1001.] | [Toxicity evaluation]
The acute effects of isoprene are related to irritation, central
nervous system depression, and asphyxia at high concentrations.
The mutagenic and genotoxic effects of isoprene seen in
in vivo and in vitro studies, as well as the carcinogenic effects
of isoprene in experimental animals (principally mice), are
believed to be due to the formation of isoprene diepoxide. |
| Questions And Answer | Back Directory | [Description]
Isoprene is a volatile colorless liquid monomer that is the basic building block of rubber,polybutadiene rubber, and polychloroprene rubber. These substances are labeled as rubber, but they differ from natural rubber. Isoprene rubber (IR) is the one synthetic rubber that is similar to natural rubber. The production of synthetic IR occurred in the mid 1950s when special organometallic catalysts for polymerizing isoprene were developed by Karl Ziegler (1898 1973) from Germany and the Italian Giulio Natta (1903 1979). Ziegler and Natta shared the 1963 Nobel Prize in chemistry for their work. Isoprene rubber has the same cis polyisoprene structure as natural rubber and has similar, but somewhat inferior, characteristics.
Rubber results from the polymerization of isoprene to form polyisoprene. The resulting structure dictates the properties of the rubber. Natural rubber has a cis 1,4 structure.This means that the carbon atoms that form the chainattach to the same side of the chain at the 1 and 4 positions. The cisstructure gives rubber its elasticity. Polyisoprene also exists in a trans 1,3 configuration. In the trans configuration, the addition takes place on opposite sides of the carbon chain.
Natural rubber occurs in a colloidal milky suspension called latex, which is obtained from numerous plants. | [Chemical Properties]
Isoprene is the monomeric unit of polyisoprene. The structure of this molecule is very similar to that of butadiene, with a simple methyl substitution differentiating the two. Although it is mainly used in the production of polyisoprene, isoprene is also used in styrene-based polymers and in butyl rubber. At room temperature it is a clear, colorless liquid with a faint odor; it is insoluble in water but soluble in acetone and other organic solvents. Unlike the chemically similar butadiene, isoprene is found in abundance in nature; for example, it is a component of natural terpenes and also is expired by plants and humans. | [References]
- https://en.wikipedia.org/wiki/Isoprene
- http://www.shell.com
- http://www.dgmk.de/petrochemistry/abstracts_content16/Reyer.pdf
- https://pubchem.ncbi.nlm.nih.gov
- https://www.lyondellbasell.com
|
|
|