| Identification | More | [Name]
Phenothiazine | [CAS]
92-84-2 | [Synonyms]
2,3:5,6-DIBENZO-1,4-THIAZINE
DIBENZO-1,4-THIAZINE
Dibenzo-p-thiazine
DIBENZOTHIAZINE
LABOTEST-BB LT00032493
PHENOTHIAZINE
PHENOXUR
THIODIPHENYLAMINE
VERMITIN
10H-Phenothiazin
10H-Phenothiazine
Afi-Tiazin
Agrazine
Antiverm
Biverm
Contaverm
Contavern
Contraverm
Danikoropa
Dibenzoparathiazine | [EINECS(EC#)]
202-196-5 | [Molecular Formula]
C12H9NS | [MDL Number]
MFCD00005015 | [Molecular Weight]
199.27 | [MOL File]
92-84-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Phenothiazine is a greenish-yellow to greenish-gray crystalline substance. Slight odor and taste. | [Melting point ]
184 °C | [Boiling point ]
371 °C(lit.)
| [density ]
1.362 | [vapor pressure ]
0.0000647 Pa (20 °C) | [refractive index ]
1.6353 | [Fp ]
202°C | [storage temp. ]
Store below +30°C. | [solubility ]
0.127mg/l | [form ]
Prills or Beads | [pka]
pKa 2.52 (Uncertain) | [color ]
Yellow | [PH]
6 (10g/l, H2O, 20℃)(aqueous suspension) | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents, strong acids. May discolour upon exposure to light. | [Water Solubility ]
2 mg/L (25 ºC) | [Sensitive ]
Light Sensitive | [Merck ]
14,7252 | [BRN ]
143237 | [Exposure limits]
ACGIH: TWA 5 mg/m3 (Skin)
NIOSH: TWA 5 mg/m3 | [InChIKey]
WJFKNYWRSNBZNX-UHFFFAOYSA-N | [LogP]
3.78 at 25℃ | [CAS DataBase Reference]
92-84-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Phenothiazine(92-84-2) | [EPA Substance Registry System]
92-84-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,N,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R43:May cause sensitization by skin contact.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R36/38:Irritating to eyes and skin .
R40:Limited evidence of a carcinogenic effect.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S29:Do not empty into drains .
S22:Do not breathe dust . | [WGK Germany ]
1
| [RTECS ]
SN5075000
| [F ]
8-23 | [Autoignition Temperature]
470 °C | [TSCA ]
Yes | [HS Code ]
29343090 | [Safety Profile]
Poison by intravenous route. Moderately toxic to humans by ingestion. Experimental reproductive effects. An insecticide. Large doses, i.e., heavy exposure, may cause hemolytic anemia and toxic degeneration of the liver. Can cause skin irritation and photosensitization. Dangerous; when heated to decomposition or on contact with acid or acid fumes it emits hghly toxic fumes of SOx and NOx. | [Hazardous Substances Data]
92-84-2(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: > 2000 mg/kg |
| Hazard Information | Back Directory | [General Description]
Light green to steel-blue powder. Acquires a greenish-brown tint under exposure to sunlight. | [Reactivity Profile]
PHENOTHIAZINE(92-84-2) is slowly decomposed by sunlight. . Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. | [Air & Water Reactions]
Insoluble in water. | [Potential Exposure]
Phenothiazine is used as an insecticide; as a base for the manufacture of tranquilizers; as anthelmintic in medicine and veterinary medicine; it is used widely as an intermediate in pharmaceutical manufacture; polymerization inhibitor, antioxidant. | [Fire Hazard]
Flash point data for this chemical are not available, but PHENOTHIAZINE is probably combustible. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. | [Shipping]
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required | [Incompatibilities]
Organosulfides are incompatible with strong acids and acid fumes; elevated temperatures; sulfur oxides and nitrogen oxides can be produced. Contact with strong reducing agents such as hydrides; azo and diazo compounds, halocarbons, isocyanates can generate heat and may form explosive hydrogen gas | [Waste Disposal]
Dissolve in combustible solvent and spray into incinerator equipped with afterburner and scrubber. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. | [Definition]
ChEBI: The 10H-tautomer of phenothiazine. | [Brand name]
Nemazine (Parke-Davis). | [Synthesis Reference(s)]
Synthesis, p. 506, 1974 DOI: 10.1055/s-1974-23359 | [Flammability and Explosibility]
Nonflammable | [Environmental Fate]
Physicochemical Properties
Phenothiazine has the standard formula S(C6H4)2NH and
includes a tricyclic structure that is related to the thiazines.
Thiazines are used in the manufacture of synthetic dies.
Chlorpromazine
Chlorpromazine is a white to off-white substance (both the base
and the hydrochloride salt) that is a powder or waxy solid as
a base and a crystalline powder as the hydrochloride. Chlorpromazine
is odorless or has a slightly amine-like odor. It has
a melting point of 56–58 °C and in the basic form is practically
insoluble in water, soluble in alcohol, and less soluble in chloroform
and ether. It is freely soluble in dilute mineral acids. As
the hydrochloride salt, chlorpromazine is soluble in water, less
soluble in alcohol and chloroform, and insoluble in ether. A
10% aqueous solution has a pH of 3.5–4.5. | [storage]
Store at -20°C | [Purification Methods]
Crystallise it from *benzene, toluene, hexane or Me2CO (charcoal) after boiling for 10minutes under reflux. Filter the crystals off and dry them in an oven at 100o, then in a vacuum desiccator over paraffin chips. Also recrystallise it twice from water and dry it in an oven at 100o for 8-10hours. It sublimes at 130o/1mm and has UV with at 253nm in heptane. [Beilstein | [Toxicity evaluation]
Phenothiazines primarily block postsynaptic neurotransmission
by binding to dopamine (D1 and D2), muscarinic, histamine H1,
and serotonergic 5-HT2 receptors. Phenothiazines also possess
peripheral adrenergic receptor blockade and quinidine-like
cardiac effects. Phenothiazines may lower the seizure threshold. |
| Questions And Answer | Back Directory | [description]
Phenothiazine is a class of agents exhibiting antiemetic, antipsychotic, antihistaminic, and anticholinergic activities. Phenothiazines antagonize the dopamine D2-receptor in the chemoreceptor trigger zone (CTZ) of the brain, potentially preventing chemotherapy-induced emesis. In addition, these agents have peripherally or centrally antagonistic activity against alpha adrenergic, serotonergic, histaminic, and muscarinic receptors.
Phenothiazines are used to treat serious mental and emotional disorders, including schizophrenia and other psychotic disorders. Some are used also to control agitation in certain patients, severe nausea and vomiting, severe hiccups, and moderate to severe pain in some hospitalized patients. Chlorpromazine is used also in the treatment of certain types of porphyria, and with other medicines in the treatment of tetanus. Phenothiazines may also be used for other conditions as determined by your doctor. | [Chemical Properties]
It is clean gray-green powder with the melting point of 185.5 ℃, boiling point of 371 ℃, 290 ℃ (5.33kPa). It is insoluble in petroleum ether, chloroform and water, and soluble in ether and hot acetic acid. It will be oxidized upon exposure to light in the air.
| [side effects]
For more than a decade, phenothiazine drugs have been used to treat a variety of disorders and have proved particularly effective in the treatment of schizophrenia. Clinical experience indicates that initial extremely high dosages are necessary to effect improvement of patients with schizophrenic illnesses.
During 1964, several sequelae have been reported following prolonged high dosage of these drugs. These recent reports refer to side effects which are apparently permanent, in contrast to earlier communications of transient deleterious effects. For example, it has been known for several years that extrapyramidal disorders occur frequently in patients taking phenothiazines; however, a reduction in dosage or cessation of medication appeared to produce a return to the normal state.
Phenothiazines may cause unwanted, unattractive, and uncontrolled face or body movements that may not go away when you stop taking the medicine. They may also cause other serious unwanted effects. You and your doctor should talk about the good this medicine will do as well as the risks of using it. Also, your doctor should look for early signs of these effects at regular visits. Your doctor may be able to stop or decrease some unwanted effects, if they do occur, by changing your dose or by making other changes in your treatment.
These medicines are available only with your doctor's prescription.
Levoprome(R) (methotrimeprazine) is no longer available in the United States. At the end of May 1998, Immunex Corporation stopped marketing it.
Once a medicine has been approved for marketing for a certain use, experience may show that it also is useful for other medical problems. Although these uses are not included in product labeling, phenothiazines are used in certain patients with the following medical conditions:
- Chronic neurogenic pain (certain continuing pain conditions)
- Huntington's chorea (hereditary movement disorder)
- Migraine headaches
| [Uses]
- Phenothiazine is a relatively widely used anthelmintic reagent with excellent efficacy in treating the Haemonchus contortus of cattle, horse and sheep, nodular worm, Bunostomum and Plasmodium chabaudi.
- Phenothiazine is the intermediates of fine chemicals such as dyes and drugs with itself being a auxiliary material for synthetic material (the anti-polymerization reagent for production of vinylon), fruit pesticides and veterinary anthelmintic.
- It is mainly used as the polymerization inhibitor for acrylic acid, acrylic esters, and methacrylic aicd as well as ester monomer.
| [Synthesis]
22 g of diphenylamine, 8.2 g of sulfur, and 3.2 gms. of anhydrous aluminum chloride are melted together. The reaction sets 140-150° C with the rapid evolution of hydrogen sulfide; by lowerg the temperature, a few degrees the reaction can be slackened. Wen the reaction has moderated, the temperature is raised to 160° C for a time. The melt, when cool, is ground up and extracted, first with water and then with dilute alcohol. The residue consists of almost pure phenothiazine. It can be recrystallised from alcohol. Yield 93%, yellowish leaflets; m.p. 180° C.
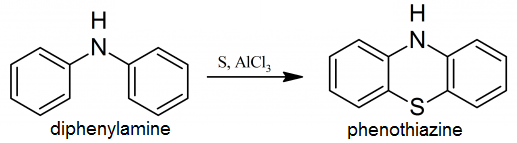
Systematic organic chemistry, by W. M. Cumming, 325-326, 1937. |
|
|