Hafniumdioxid Chemische Eigenschaften,Einsatz,Produktion Methoden
S-Sätze Betriebsanweisung:
S22:Staub nicht einatmen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
Beschreibung
Hafnium is a shiny, silvery, ductile metal and resistant to corrosion. The physical properties of hafnium metal samples are markedly affected by zirconium impurities, especially the nuclear properties, as these two elements are among the most difficult to separate because of their chemical similarity. Hafnia is used in optical coatings and as a high-k dielectric in dynamic randomaccess memory (DRAM) capacitors. Hafnium (IV) oxide is a colourless, inert solid and has been reported as one of the most common and stable compounds of hafnium. It is an electrical insulator. Hafnium dioxide is an intermediate in some processes that give hafnium metal. It reacts with strong acids and strong bases. It dissolves slowly in hydrofluoric acid. At high temperatures, it reacts with chlorine in the presence of graphite or carbon tetrachloride and forms the hafnium tetrachloride. Hafnium-based oxides are currently important materials to replace silicon oxide as a gate insulator because of its high dielectric constant. Hafnium (Hf) is found in association with zirconium ores, production based on zircon (ZrSiO4) concentrates which contain 0.5%–2% hafnium. Hafnium has extensive applications in industries especially because of its resistance to corrosion. Different compounds of hafnium used in ceramics industry are hafnium boride, hafnium carbide, hafnium nitride, hafnium oxide, hafnium silicate, and hafnium titanate. Hafnium-based oxides are currently leading candidates to replace silicon oxide as a gate insulator in fieldeffect transistors.
Chemische Eigenschaften
Hafnium oxide is a white cubic crystal, insoluble in water, hydrochloric acid, nitric acid and other common inorganic acids, and slowly dissolves in hydrofluoric acid to form fluorohafnate. It reacts with hot concentrated sulfuric acid or bisulfate to produce hafnium sulfate. Mix and heat with carbon in the presence of chlorine to obtain hafnium tetrachloride. It reacts with potassium fluorosilicate to produce potassium fluorohafnate.
Physikalische Eigenschaften
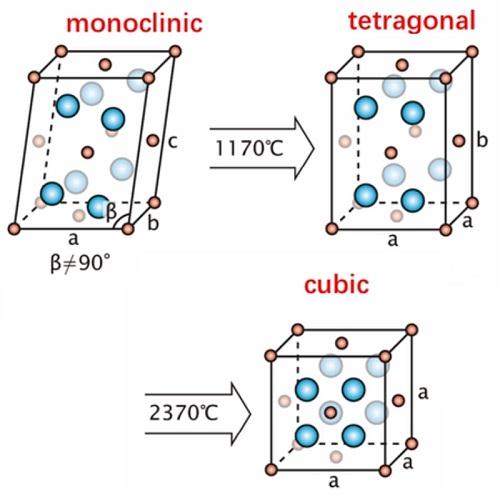
Hafnium oxide (HfO2) is a white crystalline powder. Pure hafnium oxide exists in three forms, one in the amorphous state and the other two in the crystalline state. When unstable compounds such as hafnium hydroxide and hafnium oxychloride are calcined at <400°C, amorphous hafnium oxide can be obtained. Continue to heat the hafnium oxide to 450-480°C, and start to transform into monoclinic crystals, and continue to heat to 1000-1650°C to gradually increase the lattice constant, and transform into a monomer of 4 hafnium oxide molecules. At 1700~1865℃, it starts to transform into tetragonal crystal system. Hafnium is a refractory metal which occurs in nature in zirconium minerals.
Verwenden
Hafnium(IV) oxide is used as Intermediates, Paint additives and coating additives, metal Products. And it is also used in optical coatings, as a refractory material in the insulation of such devices as thermocouples.
mögliche Exposition
Hafnium metal has been used as a
control rod material in nuclear reactors. Thus, those
engaged in fabrication and machining of such rods may be
exposed.
Versand/Shipping
UN1326 Hafnium powder, wetted with not
<,25% water (a visible excess of water must be present)
(1) mechanically produced, particle size<53 μm; (2)
chemically produced, particle size<840 μm, Hazard Class:
4.1; Labels: 4.1-Flammable solid. UN2545 Hafnium pow der, dry, Hazard Class: 4.1; Labels: 4.1-Flammable solid.
UN1346 Hafnium powder, wetted with not less than 25%
water (a visible excess of water must be present)
(1) mechanically produced, particle size less than 53 μm;
(2) chemically produced, particle size less than 840 μm,
Hazard Class: 4.1; Labels: 4.1-Flammable solid.
Inkompatibilitäten
Fine powder or dust may form explosive
mixture in air. The powder is highly flammable and a strong
reducing agent. The powder or dust reacts with moisture
forming flammable hydrogen gas; may spontaneously ignite
on contact with moist air; and at higher temperatures, with
nitrogen, phosphorous, oxygen, halogens, and sulfur; contact
with hot nitric acid; heat, shock, friction, strong oxidizers;
or ignition sources may cause explosions.
Waste disposal
Recovery. Consider recycling,
otherwise, this chemical must be disposed of in compliance
with existing federal and local regulations.
Hafniumdioxid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

