CAL-101 Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Idelalisib is an orally available selective and potent phosphatidylinositol
3-kinase δ (PI3 Kδ) inhibitor originally developed
by Calistoga Pharmaceuticals, which was acquired by Gilead in
April 2014. In July 2014, the drug was approved in the USA for
the treatment of relapsed chronic lymphocytic leukemia as well
as several oncology orphan drug designations. Since idelalisib
specifically inhibits PI3Kd, which is expressed primarily in bloodcell
lineages, the therapeutic effect is localized, limiting interference
with PI3K isoform signaling that is critical to normal function
of healthy cells.
Indications
Among the large groups of structural diverse lipid kinase inhibitors, especially against PI3Ks, idelalisib (Zydelig(R), Gilead Sciences) is the only inhibitor approved by FDA for the treatment of patients with relapsed chronic lymphocytic leukemia in combination with rituximab and patients with relapsed follicular B-cell non-Hodgkin lymphoma or small lymphocytic lymphoma.
Trademarks
Zydelig
Synthese
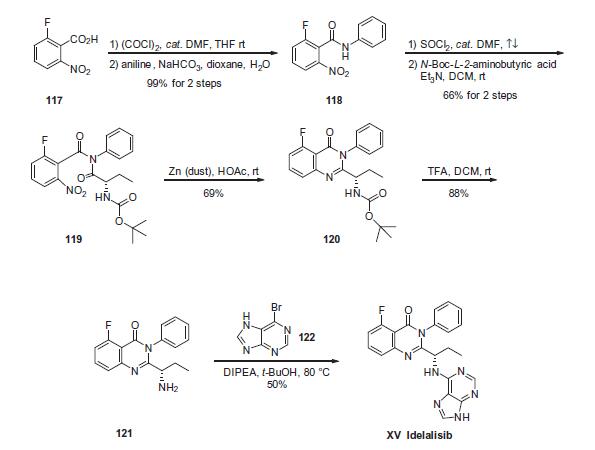
Commercial 2-fluoro-6-nitrobenzoic acid (117) was treated
with oxalyl chloride in the presence of catalytic amount of N,Ndimethylformamide
(DMF) in DCM to give the corresponding 2-
fluoro-6-nitrobenzoyl chloride as a brown syrup, which was subsequently
coupled with aniline under Schotten-Baumann conditions
to yield 2-fluoro-6-nitro-N-phenylbenzamide 118 in 99% yield.
Coupling of 118 with commercial N-Boc-2(S)-aminobutyric acid
in the presence of Et3N in DCM generated imide 119 in 66% yield.
Reductive cyclization of nitro imide 119 by means of zinc dust in
acetic acid gave the cyclized quinazolinone 120 in 69% yield, which
underwent immediate N-deprotection with TFA in DCM to furnish
the corresponding free amine 121. Finally, a substitution reaction
involving amine 121 and 6-bromopurine (122) in the presence of
DIPEA in t-BuOH gave idelalisib (XV) as a solid in 50% yield.
CAL-101 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

