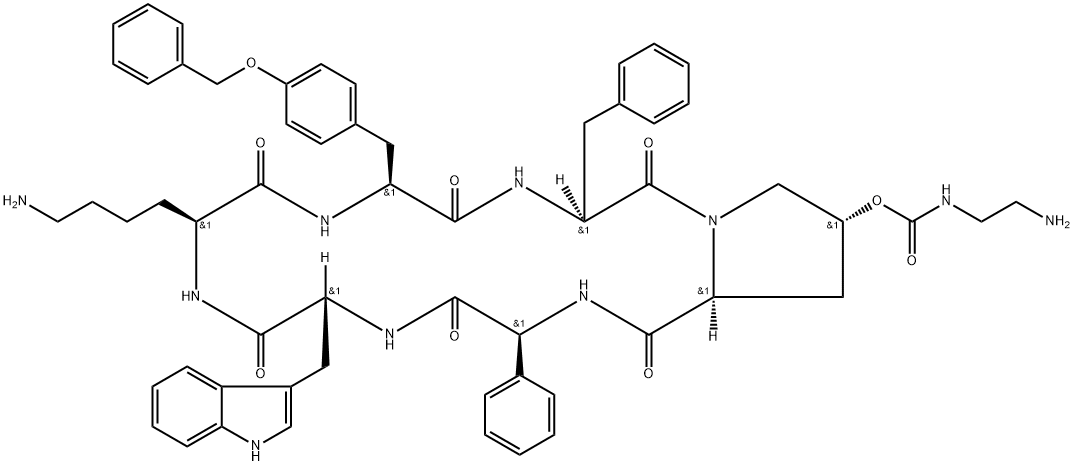
PASIREOTIDE
- CAS No.
- 396091-73-9
- Chemical Name:
- PASIREOTIDE
- Synonyms
- SOM 320;SOM 230;Signifor;PASIREOTIDE;SOM 230;SOM 320;Pasireotide (SOM-230);Pasireotide Acetate(net);CYCLO((4R)-4-(2-AMINOETHYLCARBAMOYLOXY)-L-PROLYL-L-PHENYLGLYCYL-D-TRYPTOPHYL-L-LYSYL-4-O-BENZYL-L-TYROSYL-L-PHENYLALANYL-);Cyclo[(2S)-2-phenylglycyl-D-tryptophyl-L-lysyl-O-(phenylmethyl)-L-tyrosyl-L-phenylalanyl-(4R)-4-[[[(2-aminoethyl)amino]carbonyl]oxy]-L-prolyl];[(3S,6S,9S,12R,15S,18S,20R)-9-(4-aminobutyl)-3-benzyl-12-(1H-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-15-phenyl-6-[(4-phenylmethoxyphenyl)methyl]-1,4,7,10,13,16-hexazabicyclo[16.3.0]henicosan-20-yl] N-(2-aminoethyl)carbamate
- CBNumber:
- CB02667889
- Molecular Formula:
- C58H66N10O9
- Molecular Weight:
- 1047.23
- MDL Number:
- MFCD08067735
- MOL File:
- 396091-73-9.mol
| Boiling point | 1351.4±65.0 °C(Predicted) |
|---|---|
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 11.86±0.46(Predicted) |
| Sequence | cyclo[Tyr(Bzl)-Phe-Hyp(Bom)-Phg-D-Trp-Lys] |
| CAS DataBase Reference | 396091-73-9 |
| FDA UNII | 98H1T17066 |
| NCI Drug Dictionary | pasireotide |
| ATC code | H01CB05 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H400-H315-H361-H410-H319 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P-P273-P391-P501-P273-P391-P501 |
PASIREOTIDE price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | P211010 | PasireotideTFASalt | 396091-73-9 | 1mg | $580 | 2021-12-16 | Buy |
| ApexBio Technology | B3596 | (E)-(1Z,3S,4Z,6R,7Z,9S,10Z,12S,13Z,15S,19R,20aS)-6-((1H-indol-3-yl)methyl)-9-(4-aminobutyl)-15-benzyl-12-(4-(benzyloxy)benzyl)-1,4,7,10,13-pentahydroxy-16-oxo-3-phenyl-3,6,9,12,15,16,18,19,20,20a-decahydropyrrolo[1,2-a][1,4,7,10,13,16]hexaazacyclooctadeci | 396091-73-9 | 5mg | $589 | 2021-12-16 | Buy |
| ApexBio Technology | B3596 | (E)-(1Z,3S,4Z,6R,7Z,9S,10Z,12S,13Z,15S,19R,20aS)-6-((1H-indol-3-yl)methyl)-9-(4-aminobutyl)-15-benzyl-12-(4-(benzyloxy)benzyl)-1,4,7,10,13-pentahydroxy-16-oxo-3-phenyl-3,6,9,12,15,16,18,19,20,20a-decahydropyrrolo[1,2-a][1,4,7,10,13,16]hexaazacyclooctadeci | 396091-73-9 | 1mg | $131 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FP110151 | Pasireotide acetate | 396091-73-9 | 500ug | $150 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FP110151 | Pasireotide acetate | 396091-73-9 | 1mg | $240 | 2021-12-16 | Buy |
PASIREOTIDE Chemical Properties,Uses,Production
Description
In April 2012, the European Commission approved pasireotide for the treatment of Cushing’s Disease (CD) in adult patients who have not responded to surgical interventionor forwhomsurgery is not anoption.Pasireotide was approved for the same indication by the US FDA in December of 2012. Pasireotide (also known as SOM230) is a cyclohexapeptide that acts as a somatostatin analogue to inhibit the release of ACTH. Somatostatins are cyclic peptides of 14 and 28 amino acids that play animportant role inregulating endocrineandexocrine release inmany tissues through an inhibitory mechanism. There are five known subtypes of somatostatin receptors (SSTRs). Natural somatostatins bind with high affinity to all five subtypes, however, their therapeutic use is limited by rapid degradation in plasma. Pasireotide arose fromefforts to identify a somatostatinmimetic with long-lasting inhibitory effects. Starting with a 14-amino acid somatostatin peptide, a systematic alanine scan revealed residues that were essential for receptor sub-type binding, including key b-turn regions and adjacent residues. Placing the key structural elements as unnatural amino acids in a cyclohexapeptide backbone gave pasireotide.
Originator
Novartis (Switzerland)
Uses
Pasireotide can be used in biological study of long-term treatment of Cushing''s disease with pasireotide, 5-yr results from open-label extension study of Phase III trial.
Definition
ChEBI: Pasireotide is a six-membered homodetic cyclic peptide composed from L-phenylglycyl, D-tryptophyl, L-lysyl, O-benzyl-L-tyrosyl, L-phenylalanyl and modified L-hydroxyproline residues joined in sequence. A somatostatin analogue with pharmacologic properties mimicking those of the natural hormone somatostatin; used (as its diaspartate salt) for treatment of Cushing's disease. It has a role as an antineoplastic agent. It is a homodetic cyclic peptide and a peptide hormone. It is a conjugate base of a pasireotide(2+).
brand name
Signifor
Clinical Use
Pasireotide, also known as SOM230, is a cyclic, hexameric peptide developed by Novartis which exhibits somatostatin-like activity as an antisecretory agent used in the treatment of Cushing’s disease. Pasireotide activates a broad range of somatostatin receptors, and in particular displays a significantly higher binding affinity for somatostatin receptors 1, 3, and 5 than its competitor somatostatin-mimic octreotide in vitro, as well as a comparable binding affinity for somatostatin receptor 2. Pasireotide is more potent than somatostatin in inhibiting the secretion of human growth hormone (HGH), glucagon, and insulin.
Synthesis
The synthesis of pasireotide is relatively straightforward, given that the chemical entity is a cyclic peptide. The most likely scalable route closely mimics that described by the discovery authors involving a series of conventional couplings and deprotection steps to arrive at a linear peptide which then underwent sequential release from solid support, macrocyclization, and a global deprotection step.
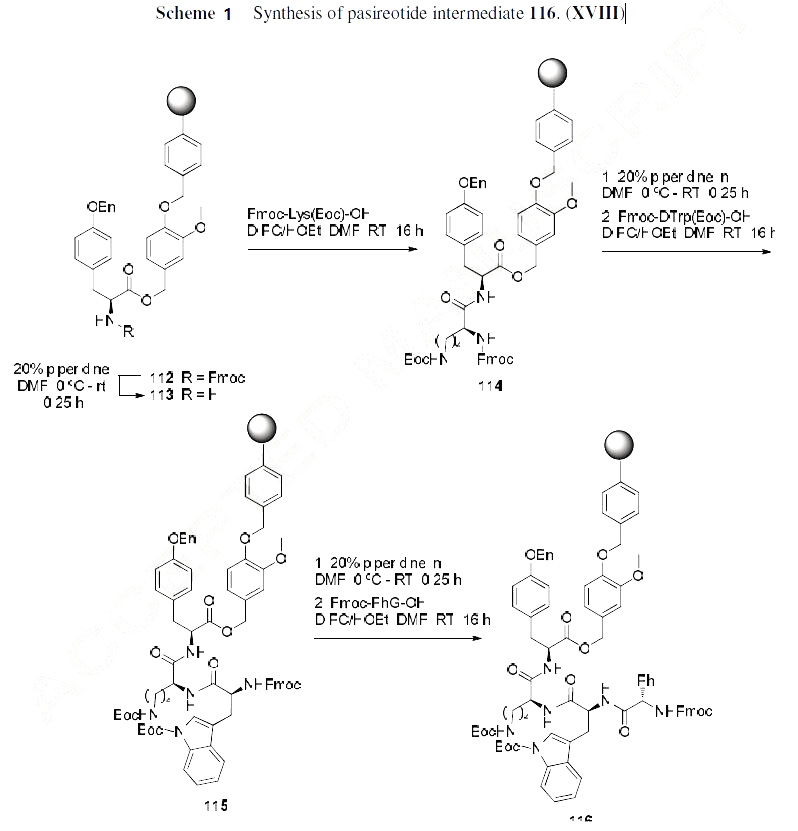
Beginning from (2S,4R)-4-hydroxyproline methyl ester (110) in the scheme above, this pyrrolidine nitrogen was first Fmoc-protected in 85% yield followed by treatment with trisphosgene and N-Boc diaminoethane to provide the prolino carbamate shown in 49% yield over the two step sequence after a recrystallization with ethyl acetate.
Next, commercially available Fmoc-Tyr(Bzl)-O-CH2-Ph(3-OCH3)-O-CH2-SASRIN157 resin (112) was used as starting material in a manually operated reactor and carried through a standard protocol consisting of repetitive cycles of N|á deprotection (piperidine/DMF, 2:8), repeated washings with DMF, and coupling using DIC/HOBT in DMF (Schemes 2 and 3). The following amino acid derivatives were sequentially coupled: Fmoc-Lys(Boc)-OH, Fmoc-D-Trp(Boc)-OH, Fmoc-PhG-OH, proline derivative 111 above, and finally Fmoc-Phe-OH. Couplings were continued or repeated until complete disappearance of residual amino groups as monitored with a ninhydrin stain test. Before cleavage of the protected linear peptide from its resin support, the Fmoc group was removed. After washings with dichloromethane, the peptide resin was transferred into a column and the peptide fragment was cleaved from solid support upon subjection to 2% TFA in dichloromethane. The eluate was immediately neutralized with a saturated NaHCO3 solution which resulted in the side chain protected fragment 119 (the Scheme) was obtained in 93% homogeneity and cyclized without further purification. For cyclization, the linear fragment was dissolved in DMF, treated with DIPEA, and then 1.5 equiv of diphenylphosphoryl azide which resulted in the protected cyclized product obtained in good yield. For complete deprotection, the residue was dissolved at 0 ??C in aqueous TFA, and the mixture was stirred at this temperature for 30 min. The product was then precipitated with ether containing ca. 10 equiv of HCl, then filtered and washed with ether, and finally dried. The entire sequence produced pasireotide (XVIII) in 20% yield from resin-bound 112.
Drug interactions
Potentially hazardous interactions with other drugs
Antifungals: avoid with ketoconazole.
Ciclosporin: possibly reduces ciclosporin
concentration.
Metabolism
Pasireotide is metabolically highly stable and in vitro
data show that pasireotide is not a substrate, inhibitor
or inducer of any major enzymes of CYP450. In healthy
volunteers, pasireotide is mainly found in the unchanged
form in plasma, urine and faeces.
Pasireotide is eliminated mainly by hepatic clearance
and is mostly found, unchanged, in the faeces (48%) and
urine.
PASIREOTIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Chinqesen Biotechnology Co., Ltd. | +86-16628886292 +86-19521323435 | sales2@qschem-pharma.com | China | 46 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Alpha Biopharmaceuticals Co., Ltd | +86-411-39042497 +8613921981412 | sales@alphabiopharm.com | China | 886 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10248 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
Related articles
- Pasireotide for Cushing's disease
- Pasireotide is a man-made protein that is similar to a hormone in the body called somatostatin. Pasireotide is used to treat ....
- Apr 29,2022
- A synthetic polypeptide drug:Pasireotide
- Pasireotide is a synthetic polypeptide analogue of somatostatin that resembles the native hormone in its ability to suppress l....
- Dec 29,2021
View Lastest Price from PASIREOTIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-10 | PASIREOTIDE
396091-73-9
|
US $70.00-700.00 / kg | 10kg | 0.99 | 20tons | Zibo Hangyu Biotechnology Development Co., Ltd | |
 |
2024-03-31 | Pasireotide
396091-73-9
|
US $40.00-20.00 / kg | 1kg | 99% | 2000000 | Shanghai Chinqesen Biotechnology Co., Ltd. | |
 |
2024-03-08 | pasireotide
396091-73-9
|
US $10.00 / kg | 1kg | 99% | 1000kg | Nantong Guangyuan Chemicl Co,Ltd |
-

- PASIREOTIDE
396091-73-9
- US $70.00-700.00 / kg
- 0.99
- Zibo Hangyu Biotechnology Development Co., Ltd
-

- Pasireotide
396091-73-9
- US $40.00-20.00 / kg
- 99%
- Shanghai Chinqesen Biotechnology Co., Ltd.
-

- pasireotide
396091-73-9
- US $10.00 / kg
- 99%
- Nantong Guangyuan Chemicl Co,Ltd