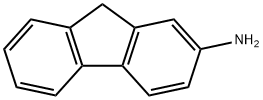
2-Aminofluorene
- CAS No.
- 153-78-6
- Chemical Name:
- 2-Aminofluorene
- Synonyms
- 2-AF;2-AminofL;Aminofluoren;2-AMinofluor;AKOS AUF02047;2-FLUORENAMINE;2-amino-fluoren;2-FLUORENEAMINE;2-AMINOFLUORENE;2-Fluoreylamine
- CBNumber:
- CB2150215
- Molecular Formula:
- C13H11N
- Molecular Weight:
- 181.23
- MDL Number:
- MFCD00001125
- MOL File:
- 153-78-6.mol
- MSDS File:
- SDS
| Melting point | 124-128 °C (lit.) |
|---|---|
| Boiling point | 304.35°C (rough estimate) |
| Density | 1.0941 (rough estimate) |
| refractive index | 1.6118 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | soluble in Ether,Alcohol |
| form | powder to crystal |
| pka | 4.34±0.20(Predicted) |
| color | White to Orange to Green |
| Water Solubility | <0.1 g/100 mL at 19.5 ºC |
| BRN | 1945861 |
| CAS DataBase Reference | 153-78-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 3A69OS195N |
| NIST Chemistry Reference | Fluoren-2-amine(153-78-6) |
| Proposition 65 List | 2-Aminofluorene |
| EPA Substance Registry System | 2-Aminofluorene (153-78-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H341-H351 | |||||||||
| Precautionary statements | P264-P270-P301+P312+P330-P501-P201-P202-P280-P308+P313-P405-P501a | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 68-40 | |||||||||
| Safety Statements | 45-36/37-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LL5075000 | |||||||||
| HS Code | 29214980 | |||||||||
| NFPA 704 |
|
2-Aminofluorene price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A55500 | 2-Aminofluorene 98% | 153-78-6 | 5g | $75.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | A55500 | 2-Aminofluorene 98% | 153-78-6 | 25g | $346 | 2023-06-20 | Buy |
| TCI Chemical | A0621 | 2-Aminofluorene >98.0%(HPLC)(T) | 153-78-6 | 5g | $56 | 2024-03-01 | Buy |
| TCI Chemical | A0621 | 2-Aminofluorene >98.0%(HPLC)(T) | 153-78-6 | 25g | $165 | 2024-03-01 | Buy |
| Alfa Aesar | B22769 | 2-Aminofluorene, 98% | 153-78-6 | 1g | $18.65 | 2024-03-01 | Buy |
2-Aminofluorene Chemical Properties,Uses,Production
Description
Occupational exposure to polycyclic aromatic amines (PAA) has
occurred historically in the rubber, textile, and dye industries.
Some sources of nonoccupational exposure to PAAs include
inhalation of tobacco smoke, emissions from heated cooking oil
and diesel engine exhaust, and dermal exposure to hair dyes.
During the 1870s, the first aromatic amine dyes were
manufactured in Germany (dyes of natural origin were used
prior to the synthesis of dyes). In 1895, a physician by the name
Rehn reported a cluster of patients who had developed bladder
cancer. He observed that all of the affected workers were
employed at a site in Germany that manufactured fuschsin dye.
The workers had all been exposed to large amounts of intermediate
arylamines. The United States first started manufacturing
dyes in the early 1900s when trade between the
United States and Germany was halted during the First World
War. DuPont was the first company to begin manufacturing
synthetic dyes in the United States, and shortly thereafter
(1930s) the physicians employed by DuPont also started
reporting an increased incidence of workers who had developed
bladder cancer. During 1947, a physician by the name of
Mengellsdorf who was employed by DuPont reported that
100% of the workers who handled the chemical betanaphthylamine
had developed bladder cancer. By the 1950s,
Chinese dye manufacturers reported the development of
bladder cancer in workers who handled benzidine. Evidence of
the development of bladder cancer associated with the manufacture
of dyes continued to mount, and during the 1970s dye
manufacturing was discontinued in the United States and was
taken over by developing nations. During the early 1970s, the
US Occupational Safety and Health Administration (OSHA)
began regulating aromatic amines that had been associated
with the development of bladder cancers. During the 1980s,
DuPont reported retrospectively that 316 of their dye
manufacturing workers had developed bladder cancer prior to
the discontinuation of dye manufacturing in the United States.
During the 1990s, the first reports of bladder cancer in the
Chinese dye manufacturing industry became public.
Hair dye products manufactured prior to the mid-1970s
contained chemicals that were shown to produce cancer in rodents. Some of these chemical included aromatic amines.
The manufacturers of hair coloring products began reformulating
their products to remove these potentially carcinogenic
compounds from their products beginning in the mid-1970s. It
is not clear if some of the ingredients in contemporary hair
products can cause an increased risk of cancer. The US National
Cancer Institute reported that there may be an increased risk of
developing non-Hodgkin’s lymphoma in people who used hair
dyes prior to the 1980s; however, the data are limited and often
inconsistent.
Chemical Properties
WHITE TO SLIGHTLY BROWN CRYSTALLINE POWDER
Uses
PAAs are used in the rubber, textile, and dye industries. They are used as intermediates in the manufacture of plastics, drugs, and carbamate pesticides. The aromatic amines 2-aminofluorene and N-acetyl aminofluorene were being developed during the 1930s for use as pesticides; however, they were found to be carcinogenic in laboratory animals. They were never marketed as pesticides.
General Description
Brown crystal powder.
Air & Water Reactions
2-Aminofluorene is sensitive to prolonged exposure to air. Insoluble in water.
Reactivity Profile
2-AMINO FLUORENE forms salts with acids and can react with oxidizing materials.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 2-Aminofluorene emits toxic fumes.
Fire Hazard
Flash point data for 2-Aminofluorene are not available, but 2-Aminofluorene is probably combustible.
Safety Profile
Suspected carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Poison by intraperitoneal route. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also AMINES.
Environmental Fate
PAAs may be transported as vapor or adsorbed onto particulates. Due to low water solubility, PAAs are not transported in water but adsorb onto soil and sediments. Leaching is negligible. Bioaccumulation is not considered a concern.
Purification Methods
Wash the amine well with H2O and recrystallise it from Et2O or 50% aqueous EtOH (25g with 400mL), and dry it in a vacuum. Store it in the dark. [Bavin Org Synth Coll Vol V 30 1973, Beilstein 12 H 1331, 12 IV 337.]
Toxicity evaluation
N-hydroxy metabolites within the gastrointestinal tract transform fluoren-2-amine into a mutagen or carcinogen. A number of PAAs are potent bladder carcinogens. As noted previously, sequential hydroxylation and glucuronidation lead to urinary excretion, with metabolites in the urinary bladder. While glucuronidation enhances excretion via the urine, a glucuronidase in the bladder hydrolyzes the glucuronide and under acidic conditions N-hydroxylarylamines are formed. Subsequent conversion of the amine leads to an aryl nitrenium ion, which can initiate tumor formation. Sulfate esters can degrade to electrophilic nitronium ion–carbonium ion, which can form adducts with macromolecules.
2-Aminofluorene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 16805 | 58 |
| Beijing Green Guardee Technology Co., Ltd | 86-010-69706062 | sales2@greenguardee.com | CHINA | 650 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| Wuhan Chemwish Technology Co., Ltd | 027-67849912 | sales@chemwish.com | CHINA | 10828 | 58 |
View Lastest Price from 2-Aminofluorene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-01-06 | 2-aminofluorene
153-78-6
|
US $188.00-1.00 / KG | 1KG | 99%, 99.5% Sublimated | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-07-29 | 2-Aminofluorene
153-78-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-02-09 | 2-Aminofluorene
153-78-6
|
US $0.00-0.00 / KG | 1KG | 99% | 20 mt | Hebei Guanlang Biotechnology Co., Ltd. |
-

- 2-aminofluorene
153-78-6
- US $188.00-1.00 / KG
- 99%, 99.5% Sublimated
- Henan Fengda Chemical Co., Ltd
-

- 2-Aminofluorene
153-78-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- 2-Aminofluorene
153-78-6
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.