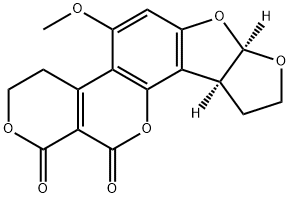
AFLATOXIN G2
- CAS No.
- 7241-98-7
- Chemical Name:
- AFLATOXIN G2
- Synonyms
- AF G2;AFLATOXIN G2;AFLATOXIN G2(RG);Dihydroaflatoxin G1;AflatoxinG2,crystalline;Aflatoxin G2;Aflatoxin G2 0.5ug/ml in ACN;Aflatoxin G2 Solution, 3 Mg/L;10a-hexahydro-5-methoxy-1(7ar-cis)-;AFLATOXIN G2 FROM ASPERGILLUS FLAVUS
- CBNumber:
- CB3291098
- Molecular Formula:
- C17H14O7
- Molecular Weight:
- 330.29
- MDL Number:
- MFCD00078141
- MOL File:
- 7241-98-7.mol
| Melting point | 237-240 °C |
|---|---|
| alpha | D -473° (c = 0.084 in chloroform) |
| Boiling point | 387.74°C (rough estimate) |
| Density | 1.3099 (rough estimate) |
| refractive index | 1.4790 (estimate) |
| Flash point | 2 °C |
| storage temp. | 2-8°C |
| solubility | DMF: Soluble; DMSO: Soluble; Ethanol: Soluble; Methanol: Soluble |
| form | White to yellow powder. Blue-green fluorescence. |
| Water Solubility | 15mg/L(temperature not stated) |
| BRN | 1692017 |
| LogP | 0.443 (est) |
| FDA UNII | 2MS0D8WA29 |
| EPA Substance Registry System | Aflatoxin G2 (7241-98-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H304-H315-H319-H340-H350-H372-H412 | |||||||||
| Precautionary statements | P210-P273-P301+P310-P303+P361+P353-P305+P351+P338-P331 | |||||||||
| Hazard Codes | T+,T,Xn,F | |||||||||
| Risk Statements | 45-26/27/28-36-20/21/22-11-39/23/24/25-23/24/25-65-48/23/24/25-36/38-46 | |||||||||
| Safety Statements | 53-28-36/37-45-36-26-16-7-62 | |||||||||
| RIDADR | UN 3462 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LV1700000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 30029090 | |||||||||
| Toxicity | LD50 orally in day old duckling: 172.5 mg/50 gm body wt (Carnaghan) | |||||||||
| NFPA 704 |
|
AFLATOXIN G2 price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A0263 | Aflatoxin G2 | 7241-98-7 | 1mg | $228 | 2024-03-01 | Buy |
| Sigma-Aldrich | CRM46326 | Aflatoxin G2 solution certifiedreferencematerial,3?μg/mLinbenzene:acetonitrile(98:2),ampuleof | 7241-98-7 | 1mL | $51.78 | 2024-03-01 | Buy |
| Sigma-Aldrich | 34033 | Aflatoxin G2 solution 0.5?μg/mLinacetonitrile,analyticalstandard | 7241-98-7 | 2ml | $339 | 2024-03-01 | Buy |
| Cayman Chemical | 11296 | Aflatoxin G2 ≥98% | 7241-98-7 | 500μg | $110 | 2024-03-01 | Buy |
| Cayman Chemical | 11296 | Aflatoxin G2 ≥98% | 7241-98-7 | 2.5mg | $388 | 2024-03-01 | Buy |
AFLATOXIN G2 Chemical Properties,Uses,Production
Chemical Properties
The aflatoxins are a group of molds produced by the fungus Aspergillus flavus. They are natural contaminants of fruits, vegetables, and grains. They are also described as a series of condensed ring heterocyclic compounds. They form colorless to pale yellow crystals. Practically insoluble in water.
Chemical Properties
yellow powder
Uses
Aflatoxin G2 is the minor analogue of the green fluorescent family of bisfuranocoumarin mycotoxins produced by Aspergillus flavus and related species. Alfatoxins are one of the most potent mycotoxins known but are in fact "pre-toxins", requiring metabolic activation to the toxic principle. Aflatoxins are found widely in nature in trace amounts, particularly in grains and nuts. The toxicity of these metabolites was first recognised in the 1950s and their structures elucidated in 1963. Aflatoxins have been extensively reviewed.
Uses
Aflatoxins B1, B2, G1, G2 as secondary metabolites of fungal species such as Aspergillus flavus or Aspergillus parasiticus growing on a variety of foods (peanuts, nuts, spices, cereals). Aflatoxins are a group of very carcinogenic mycotoxins with hepatotoxic effects.
Definition
ChEBI: Aflatoxin G2 is a member of coumarins.
General Description
Very light and fluffy crystalline solid. Exhibits green-blue fluorescence.
Air & Water Reactions
Slightly water soluble
Reactivity Profile
AFLATOXIN G2 is incompatible with strong oxidizing agents, strong acids and strong bases.
Health Hazard
ACUTE/CHRONIC HAZARDS: AFLATOXIN G2 is extremely toxic. Ingestion of even microgram quantities can cause toxic affects and, possibly, death. It can also be absorbed through the skin. Allow only your most experienced personnel to work with AFLATOXIN G2. All non-essential personnel should leave the laboratory.
Fire Hazard
Flash point data for AFLATOXIN G2 are not available. AFLATOXIN G2 is probably combustible.
Biochem/physiol Actions
Hepatocarcinogen. Food contaminant produced by Aspergillus flavus, a common soil fungus.
Potential Exposure
Aflatoxins are a group of toxic metabolites produced by certain types of fungi. Aflatoxins are not commercially manufactured; they are naturally occurring contaminants that are formed by fungi on food during conditions of high temperatures and high humidity. Most human exposure to aflatoxins occurs through ingestion of contaminated food. The estimated amount of aflatoxins that Americans consume daily is estimated to be 0.15 0.50 μg. Grains, peanuts, tree nuts, and cottonseed meal are among the more common foods on which these fungi grow. Meat, eggs, milk, and other edible products from animals that consume aflatoxincontaminated feed may also contain aflatoxins. Aflatoxins can also be breathed in
Shipping
UN3172 Toxins, extracted from living sources, solid or liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Use of oxidizing agents, such as hydrogen peroxide or 5% sodium hypochlorite bleach. Acids and bases may also be used.
AFLATOXIN G2 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32686 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | scglp@glp-china.com | CHINA | 1824 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 | ada@ipurechemical.com | CHINA | 3465 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9634 | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | Ryan@jiutian-bio.com | China | 7433 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33350 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
7241-98-7(AFLATOXIN G2)Related Search:
1of4