technetium
- CAS No.
- 7440-26-8
- Chemical Name:
- technetium
- Synonyms
- technetium;technetium atom;technetium ISO 9001:2015 REACH
- CBNumber:
- CB3932555
- Molecular Formula:
- Tc
- Molecular Weight:
- 98.906265
- MDL Number:
- MOL File:
- 7440-26-8.mol
- MSDS File:
- SDS
| Melting point | 2250 ±50° |
|---|---|
| Boiling point | 4265°C (estimate) |
| Density | 11.000 |
| form | hexagonal crystals |
| color | hexagonal, hexane crystals, crystalline |
| FDA UNII | NC93A173TP |
| EPA Substance Registry System | Technetium (7440-26-8) |
| Poissons Ratio | 0.31 |
|---|---|
| Shear Modulus | 123 GPa |
| Hardness, Vickers | 151 |
| Bulk Modulus | 281 GPa |
| Hardness, Brinell | 112 |
technetium Chemical Properties,Uses,Production
Description
Element with atomic number 43, group VIIB of the periodic table, aw 98.9062, valences of 4, 5, 6, 7; three radioactive isotopes of half-life more than 105 years, also several of relatively short half-life, some of which are β emitters. Technetium was first obtained by the deuteron bombardment of molybdenum, but since has been found in the fission products of uranium and plutonium. The chemistry of technetium has been studied by tracer techniques and is similar to that of rhenium and manganese. The free metal is obtained from reactor fission products by solvent extraction followed by crystallization as ammonium pertechnetate, which is reduced with hydrogen. The metal is silver-gray in appearance, mp 2200C (4000F), d 11.5, slightly magnetic. Compounds of the types TcO2, Tc2O7, NH4TcO4, etc. have been prepared. The pertechnetate ion has strong anticorrosive properties. Technetium and its alloys are superconductors and can be used to create high-strength magnetic fields at low temperature. Tc-99 (metastable) is the mostwidely used isotope in nuclear medicine.
Uses
Metallurgical tracer, cryochemistry, corrosion resistance, nuclear medicine.
Chemical Properties
closed-packed hexagonal, a=0.2741 nm, c=0.4399nm; enthalpy of sublimation 650 kJ/mol; enthalpy of vaporization ~577 kJ/mol; enthalpy of fusion 33.29 kJ/mol; slowly tarnishes in moist air; when obtained from H2 reduction of ammonium pertechnate, has silvery gray color, and a spongy mass; resembles rhenium in chemical behavior; Debye constant 455K; used as a metallurgical tracer, in nuclear medicine, and to protect against corrosion [HAW93] [MER06] [RAR83] [CRC10]
Physical properties
As the central member of the triad of metals in group 7, technetium (period 5) has similarphysical and chemical properties as its partners manganese (period 4) above it and rhenium(period 6) below it. The sizes of their atomic radii do not vary greatly: Mn = 127, Tc = 136,and Re = 137. Neither does their level of electronegativity vary significantly: Mn = 1.5, Tc =1.9, and Re = 1.9.
Technetium metal is grayish-silver and looks much like platinum. As with most transitionelements, technetium in pure form is a noncorrosive metal. It requires only 55 ppm of technetiumadded to iron to transform the iron into a noncorroding alloy. Because of technetium’sradioactivity, its use as an alloy metal for iron is limited so as to not expose humans to unnecessaryradiation.
Technetium’s melting point is 2,172°C, its boiling point is 4,877°C, and its density is11.50 g/cm3 .
Isotopes
There are 47 isotopes. None are stable and all are radioactive. Most are producedartificially in cyclotrons (particle accelerators) and nuclear reactors. The atomicmass of its isotopes ranges from Tc-85 to Tc-118. Most of technetium’s radioactiveisotopes have very short half-lives. The two natural radioisotopes with the longest halflives—Tc-98 = 4.2×10+6 years and Tc-99 = 2.111×10+5 years—are used to establishtechnetium’s atomic weight.
Origin of Name
Technetium’s name was derived from the Greek word technetos, meaning “artificial.”
Occurrence
Technetium is the 76th most abundant element, but it is so rare that it is not found as astable element on Earth. All of it is artificially produced. Even though natural technetium isso scarce that it is considered not to exist on Earth, it has been identified in the light spectrumfrom stars. Using a spectroscope that produces unique lines for each element, scientists areable to view several types of stars. The resulting spectrographs indicate that technetium existsin the stars and thus the universe, but not on Earth as a stable element.
It was the first new element to be produced artificially from another element experimentallyin a laboratory. Today, all technetium is produced mostly in the nuclear reactors of electricalgeneration power plants. Molybdenum-98 is bombarded with neutrons, which then becomesmolybdenum-99 when it captures a neutron. Since Mo-99 has a short half-life of about 66hours, it decays into Tc-99 by beta decay.
History
Element 43 was predicted on the basis of the periodic table, and was erroneously reported as having been discovered in 1925, at which time it was named masurium. Technetium was actually discovered by Perrier and Segre in Italy in 1937. It was found in a sample of molybdenum that was bombarded by deuterons in the Berkeley cyclotron, and which E. Lawrence sent to these investigators. Technetium was the first element to be produced artificially. Since its discovery, searches for the element in terrestrial materials have been made without success. If it does exist, the concentration must be very small. Technetium has been found in the spectrum of S-, M-, and N-type stars, and its presence in stellar matter is leading to new theories of the production of heavy elements in the stars. Forty-three isotopes and isomers of technetium, with mass numbers ranging from 86 to 113, are known. 97Tc has a half-life of 2.6 × 106 years. Tc has a half-life of 4.2 × 106 years. The isomeric isotope 95mTc, with a half-life of 61 days, is useful for tracer work, as it produces energetic gamma rays. Technetium metal has been produced in kilogram quantities. The metal was first prepared by passing hydrogen gas at 1100°C over Tc2S7. It is now conveniently prepared by the reduction of ammonium pertechnetate with hydrogen. Technetium is a silvery-gray metal that tarnishes slowly in moist air. Until 1960, technetium was available only in small amounts and the price was as high as $2800/g, but the price is now of the order of $100/g. The chemistry of technetium is similar to that of rhenium. Technetium dissolves in nitric acid, aqua regia, and concentrated sulfuric acid, but is not soluble in hydrochloric acid of any strength. The element is a remarkable corrosion inhibitor for steel. It is reported that mild carbon steels may be effectively protected by as little as 55 ppm of KTcO4 in aerated distilled water at temperatures up to 250°C. This corrosion protection is limited to closed systems, since technetium is radioactive and must be confined. 99Tc has a specific activity of 6.2 × 108 Bq/g. Activity of this level must not be allowed to spread. 99Tc is a contamination hazard and should be handled in a glove box. The metal is an excellent superconductor at 11K and below.
Characteristics
Technetium was the first element, not found on Earth, to be artificially produced by bombardingmolybdenum with deuterons.
The major characteristic of technetium is that it is the only element within the 29 transitionmetal-to-nonmetal elements that is artificially produced as a uranium-fission product innuclear power plants. It is also the lightest (in atomic weight) of all elements with no stableisotopes. Since all of technetium’s isotopes emit harmful radiation, they are stored for sometime before being processed by solvent extraction and ion-exchange techniques. The two longlivedradioactive isotopes, Tc-98 and Tc-99, are relatively safe to handle in a well-equippedlaboratory.
Since all of technetium’s isotopes are produced artificially, the element’s atomic weight(atomic mass units) is determined by which isotopes are selected for the calculation.
Uses
Technetium is one of the few artificially produced elements that has practical industrial applications.One is that a very small amount (55-ppm) added to iron creates a corrosion-resistantalloy metal. This property is shared with many of the other transition metallic elements, but notwith other artificially produced elements that have higher atomic numbers and are radioactive.
A radioisotope of technetium is widely used in nuclear medicine. The patient is injectedwith saline solution containing Tc-99m (the superscript “m” means that the isotope is unstableand that its nuclei holds more energy than the regular Tc-99 nuclei into which it decays). Thismeans that the Tc-99m will start to emit energy and will finally decay and change to the regularnuclei of Tc-99 when injected into the patient. This energy is in the form of very penetratinggamma rays (a strong type of X-rays). The radioactive solution of Tc-99m may be combinedwith other elements that are absorbed by certain organs of the human body being diagnosedor treated. For instance, adding tin to the solution targets the red blood cells, whereas phosphorusin the solution concentrates the radioactive solution in heat muscles. The gamma raysare strong enough to expose an X-ray film that depicts the internal image of the organ underexamination. This procedure is safe because Tc-99m has a half-life of only 6.015 hours, andthe Tc-99 has a half-life of over 200,000 years. However, the radioactivity will be harmless inless than a day because the body rapidly eliminates the residual radioactive solution.
Technetium is also used as an alloy metal to produce super-strong magnets that are supercooledto near absolute zero to improve their efficiency. Powerful magnets are used in imagingequipment and possibly in future magnetic driven trains. Its radioactivity makes it useful as atracer in the production of metals and tracing flowing fluids in pipelines.
Uses
Minute quantities of TcO4- ion exert remarkable inhibition of the corrosion of soft iron in neutral aqueous solution: Cartledge, J. Am. Chem. Soc. 77, 2658 (1955).
Preparation
Technetium isotopes are prepared by bombardment of molybdenum with protons and neutrons. A few nuclear reactions are shown for the three longlived isotopes:
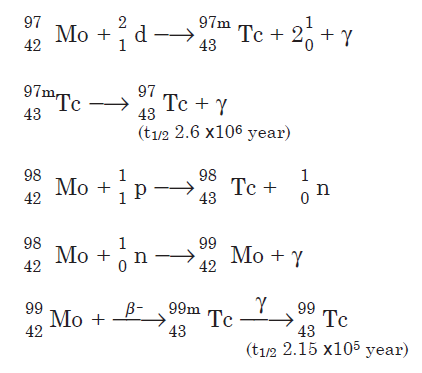
Technetium-99 also is a fission product of uranium-235.
Pure technetium metal may be prepared by reducing ammonium pertechnate, NH4TcO4, with hydrogen at high temperatures. Hydrogen reduction at about 200°C first forms the oxide, TcO2, which is reduced to Tc metal at 600 to 800°C.
Definition
technetium: Symbol Tc. A radioactivemetallic transition element;a.n. 43; m.p. 2172°C; b.p. 4877°C. Theelement can be detected in certainstars and is present in the fissionproducts of uranium. It was firstmade by Carlo Perrier and EmilioSegré (1905–89) by bombardingmolybdenum with deuterons to givetechnetium–97. The most stable isotopeis technetium–99 (half-life 2.6 ×106 years); this is used to some extentin labelling for medical diagnosis.There are sixteen known isotopes.Chemically, the metal has propertiesintermediate between manganeseand rhenium.
Definition
A transition metal that does not occur naturally on Earth. It is produced artificially by bombarding molybdenum with neutrons and also during the fission of uranium. It is radioactive. Symbol: Tc; m.p. 2172°C; b.p. 4877°C; r.d. 11.5 (est.); p.n. 43; r.a.m. 98.9063 (99Tc); most stable isotope 98Tc (half-life 4.2 × 106 years).
Hazard
The hazards of technetium are the same as for all radioactive elements. Excessive exposureto radiation can cause many kinds of tissue damage—from sunburn to radiation poisoningto death.
technetium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9126 | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | Ryan@jiutian-bio.com | China | 7433 | 58 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 58 |





