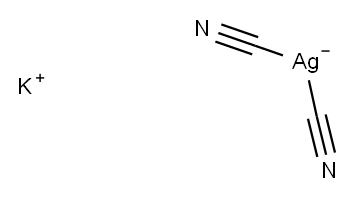
Potassium dicyanoargentate
- CAS No.
- 506-61-6
- Chemical Name:
- Potassium dicyanoargentate
- Synonyms
- s900;rcrawastenumberp099;Kaliumdicyanoargentat;kyanostribrnandraselny;Potassiumcyanoargenate;potassiumargentocyanide;SILVER POTASSIUM CYANIDE;POTASSIUM CYANOARGENTATE;POTASSIUM SILVER CYANIDE;dicyano-argentates(i)(sol
- CBNumber:
- CB5123490
- Molecular Formula:
- C2AgN2.K
- Molecular Weight:
- 199
- MDL Number:
- MFCD00036297
- MOL File:
- 506-61-6.mol
| Density | 2.36 |
|---|---|
| storage temp. | Poison room |
| solubility | soluble in H2O |
| form | Powder |
| color | White |
| Water Solubility | soluble |
| Sensitive | Light Sensitive |
| Merck | 14,7669 |
| Stability | Stable, but light sensitive. Contact with acids liberates very toxic gas. |
| CAS DataBase Reference | 506-61-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 46KRR09TKY |
| EPA Substance Registry System | Potassium silver cyanide (506-61-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H315-H318-H410 | |||||||||
| Precautionary statements | P262-P273-P280-P302+P352+P310-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T+,N,Xi | |||||||||
| Risk Statements | 26/27/28-32-50/53-36/37/38 | |||||||||
| Safety Statements | 7-1/2-29-45-61-60-37/39-28A-26 | |||||||||
| RIDADR | UN 1588 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TT5775000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28432900 | |||||||||
| NFPA 704 |
|
Potassium dicyanoargentate Chemical Properties,Uses,Production
Chemical Properties
Potassium silver cyanide is a white crystalline solid.
Chemical Properties
Potassium silver cyanide is a poisonous, white solid made of crystals, which are light sensitive. It is soluble in water and acids and slightly soluble in ethanol. It emits very toxic fumes when heated to decomposition.
Uses
In silver plating.
Uses
Silver platingPotassium silver cyanide is used in silver plating and in the manufacturing of antiseptics. It is a versatile bridging ligand and finds use for multidimensional polymer construction.
General Description
White crystals. Poisonous. Used in silver plating, as a bactericide and in the manufacture of antiseptics. Not registered as a pesticide in the U.S.
Air & Water Reactions
Water soluble [Merck].
Reactivity Profile
Potassium dicyanoargentate is light sensitive. Acids precipitate silver cyanide from its aqueous solution. Fusion with metal chlorates, perchlorates, nitrates or nitrites can cause violent explosions [Bretherick 1979 p. 101].
Hazard
Very toxic.
Health Hazard
The primary health hazard is as a cyanide. (Non-specific -- Cyanide, Inorganic, n.o.s.). It is poisonous and may be fatal if inhaled, swallowed or absorbed through the skin. Fire may produce irritating or poisonous gases.
Health Hazard
Potassium silver cyanide is a poisonous, white solid made of crystals, which are light sensitive. It is soluble in water and acids, and slightly soluble in ethanol. It emits very toxic fumes when heated to decomposition. Synonyms for potassium silver cyanide are potassium argentocyanide and potassium dicyanoargentate
Fire Hazard
When heated to decomposition, Potassium dicyanoargentate emits very toxic fumes of cyanide and nitrogen oxides. Avoid light.
Flammability and Explosibility
Not classified
Safety Profile
Poison by ingestion. A severe skin and eye irritant. When heated to decomposition it emits very toxic fumes of CN-, K2O, and NOx. See also CYANIDE and SILVER COMPOUNDS.
Potential Exposure
Potassium silver cyanide is used in silver plating; as a bactericide; and in the manufacture of antiseptics. Not registered for use as a pesticide in the United States
Shipping
UN1588 Cyanides, inorganic, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Contact with acid, acid fumes release hydrogen cyanide. Incompatible with water, steam, or when heated to decomposition, emits toxic and flammable cyanide vapors. Potassium silver cyanide reacts with carbon dioxide releasing hydrogen cyanide. Light sensitive. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materi- als, strong bases, strong acids, oxoacids, epoxides. Acids precipitate silver cyanide from its aqueous solution. Fusion with metal chlorates, perchlorates, nitrates or nitrites can cause violent explosions
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations govern- ing storage, transportation, treatment, and waste disposal.