ChemicalBook >> CAS DataBase List >>4-METHOXY-3-NITROACETOPHENONE
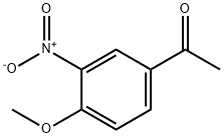
4-METHOXY-3-NITROACETOPHENONE
- CAS No.
- 6277-38-9
- Chemical Name:
- 4-METHOXY-3-NITROACETOPHENONE
- Synonyms
- ASISCHEM D29251;Einecs 228-476-7;3-NITRO-4-METHOXYACETOPHENONE;4-METHOXY-3-NITROACETOPHENONE;4'-METHOXY-3'-NITROACETOPHENONE;4'-Methoxy-3'-nitroacetophenone;1-(4-METHOXY-3-NITROPHENYL)ETHANONE;4'-Methoxy-3'-nitroacetophenone 98%;4'-Methoxy-3'-nitroacetophenone, 98+%;1-(4-METHOXY-3-NITROPHENYL)ETHAN-1-ONE
- CBNumber:
- CB5702714
- Molecular Formula:
- C9H9NO4
- Molecular Weight:
- 195.17
- MDL Number:
- MFCD00017023
- MOL File:
- 6277-38-9.mol
- MSDS File:
- SDS
Last updated:2023-04-23 13:52:06
| Melting point | 97-100 °C(lit.) |
|---|---|
| Boiling point | 315.1±22.0 °C(Predicted) |
| Density | 1.244±0.06 g/cm3(Predicted) |
| form | powder to crystal |
| color | White to Yellow to Green |
| BRN | 646069 |
| CAS DataBase Reference | 6277-38-9(CAS DataBase Reference) |
| FDA UNII | EU9GL6H9FT |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319 | |||||||||
| Precautionary statements | P261-P302+P352-P305+P351+P338 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 2914790090 | |||||||||
| NFPA 704 |
|
4-METHOXY-3-NITROACETOPHENONE price More Price(24)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 553654 | 4′-Methoxy-3′-nitroacetophenone 97% | 6277-38-9 | 10g | $50.76 | 2024-03-01 | Buy |
| TCI Chemical | M2811 | 4'-Methoxy-3'-nitroacetophenone >98.0%(GC) | 6277-38-9 | 5g | $43 | 2024-03-01 | Buy |
| Alfa Aesar | L04149 | 4'-Methoxy-3'-nitroacetophenone, 98+% | 6277-38-9 | 50g | $225 | 2021-12-16 | Buy |
| Alfa Aesar | L04149 | 4'-Methoxy-3'-nitroacetophenone, 98+% | 6277-38-9 | 10g | $63.8 | 2021-12-16 | Buy |
| TRC | M265778 | 1-(4-Methoxy-3-nitrophenyl)ethanone | 6277-38-9 | 500mg | $75 | 2021-12-16 | Buy |
4-METHOXY-3-NITROACETOPHENONE Chemical Properties,Uses,Production
Uses
4′-Methoxy-3′-nitroacetophenone may be used to synthesize dimethylamino compound, via W2 Raney nickel catalyzed reductive methylation.
Preparation
Preparation by reaction of acetyl chloride with 2-nitroanisole in the presence of aluminium chloride,
? in nitrobenzene at 0° ;
? in carbon disulfide.
General Description
4′-Methoxy-3′-nitroacetophenone can be synthesized from p-methoxyacetophenone via nitration.
4-METHOXY-3-NITROACETOPHENONE Preparation Products And Raw materials
Raw materials
Preparation Products
4-METHOXY-3-NITROACETOPHENONE Suppliers
Global( 87)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Fluoropharm Co., Ltd. | +86-0571-85586753; +8613336034509 | sales@fluoropharm.com | China | 1377 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 | dj@sgtlifesciences.com | China | 12382 | 58 |
| Aromsyn Co., Ltd. | +86-0571-85585865 +8613336024896 | CB@aromsyn.com | China | 19185 | 58 |
| Nanjing Bicbiotechnology Co., Ltd | +86-2552131256 +86-18251840740 | sales@bicbiotech.com | China | 6000 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| Alfa Aesar | 400-6106006 | saleschina@alfa-asia.com | China | 30132 | 84 |
| Energy Chemical | 021-021-58432009 400-005-6266 | sales8178@energy-chemical.com | China | 44751 | 61 |
| Shanghai Hanhong Scientific Co.,Ltd. | China | 42982 | 64 |
6277-38-9(4-METHOXY-3-NITROACETOPHENONE)Related Search:
4-METHOXY-3-NITROACETOPHENONE
4-Benzyloxy-3-nitroacetophenone
(2-FLUOROPHENYL)(4-METHOXY-3-NITROPHENYL)METHANONE, 95
5-ETHYL-2-METHOXY-3-NITROACETOPHENONE
1-[4-[(3,4-DICHLOROBENZYL)OXY]-3-NITROPHENYL]ETHAN-1-ONE
4-(4-FLUOROBENZYLOXY)-3-NITROACETOPHENONE
3-CHLORO-4'-METHOXY-3'-NITROBENZOPHENONE
2-Methoxy-5-Nitroacetophenone
3'-METHOXY-4'-NITROACETOPHENONE,3-METHOXY-4-NITROACETOPHENONE
5-FLUORO-2-METHOXY-3-NITROACETOPHENONE
1of4
ASISCHEM D29251
1-(4-METHOXY-3-NITROPHENYL)ETHAN-1-ONE
1-(4-METHOXY-3-NITROPHENYL)ETHANONE
3-NITRO-4-METHOXYACETOPHENONE
4'-METHOXY-3'-NITROACETOPHENONE
4-METHOXY-3-NITROACETOPHENONE
Einecs 228-476-7
4'-Methoxy-3'-nitroacetophenone 98%
1-(4-Methoxy-3-nitrophenyl)ethan-1-one, 4-Acetyl-2-nitroanisole
4'-Methoxy-3'-nitroacetophenone, 98+%
JRH-08503, 1-(4-Methoxy-3-nitrophenyl)ethanone, 97%
Ethanone, 1-(4-methoxy-3-nitrophenyl)-
4'-Methoxy-3'-nitroacetophenone
6277-38-9
CH3OC6H3NO2COCH3
CH3OO2NC6H3COCH3
Organic Building Blocks
Ketones
Carbonyl Compounds
C9
Building Blocks
Aromatic Acetophenones & Derivatives (substituted)
C9
Carbonyl Compounds
Ketones