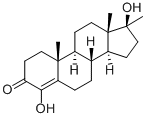
Oxymesterone
- CAS No.
- 145-12-0
- Chemical Name:
- Oxymesterone
- Synonyms
- Aranabol;Anamidol;ORANABOL;Theranabol;Oxymestrone;OXYMESTERONE;4-HYDROXY-METHYLTESTOSTERONE;4-Hydroxy-17-methyltestosterone;Testosterone, 4-hydroxy-17-methyl-;4-HYDROXY-17ALPHA-METHYLTESTOSTERONE
- CBNumber:
- CB6364959
- Molecular Formula:
- C20H30O3
- Molecular Weight:
- 318.45
- MDL Number:
- MFCD00273178
- MOL File:
- 145-12-0.mol
- MSDS File:
- SDS
| Melting point | 166-169°C |
|---|---|
| alpha | D20 +69° (ethanol) |
| Boiling point | 475.6±45.0 °C(Predicted) |
| Density | 1.17±0.1 g/cm3(Predicted) |
| storage temp. | Controlled Substance, -20°C Freezer, Under Inert Atmosphere |
| solubility | Acetonitrile (Very Slightly), Chloroform (Slightly), Ethanol (Slightly), Methano |
| form | Solid |
| pka | 9.32±0.70(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 145-12-0(CAS DataBase Reference) |
| FDA UNII | 4R73K9MRMX |
| NIST Chemistry Reference | Oxymesterone(145-12-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501 |
| DEA Controlled Substances | CSCN: 4000 CSA SCH: Schedule III NARC: No |
Oxymesterone price
Oxymesterone Chemical Properties,Uses,Production
Chemical Properties
White Solid
Originator
Anamidol,Iwaki
Uses
An anabolic steroid.
Definition
ChEBI: Oranabol is a 3-hydroxy steroid. It has a role as an androgen.
Manufacturing Process
2 Methods of producing of 4-hydroxy-17α-methyltestosterone:
1. A solution of 1.0 g of crude 4,5-oxido-17α-methyltestosterone in 50 ml of methanol is allowed to stand at room temperature overnight with 10 ml of water and 1 ml of concentrated sulfuric acid. It is then poured into water containing sodium chloride and extracted three times with ethyl acetate. The solvent is washed with water, then with 10% sodium bicarbonate solution and again with water to neutrality. The residue remaining after evaporation of the solvent is crystallized from methanol, giving 17α-methyl-androstane4β,5α,17β-triol-3-one with a melting point of 203°-205°C.
A solution of 0.22 g of 17α-methyl-androstane-4β,5α,17β-triol-3-one in 100 ml of methanol is allowed to stand at room temperature for 22 h, under nitrogen, with 0.30 g of potassium hydroxide in 4 ml of water and 20 ml of methanol. The solution is then neutralized with acetic acid, concentrated in vacuo, diluted with water and extracted three times with ethyl acetate. The extract is washed with water and the solvent removed by distillation. The remaining residue is chromatographed over Florisil 30-60 mesh. The fractions eluted with benzene and benzene-ether (10:1) are combined and by crystallization from ether-petroleum ether give 4-hydroxy-17α-methyltestosterone (0.120 g) melting at 168°-170°C.
2. A solution of 20.0 g of 17α-testosterone in 500 ml of trimethylcarbinol is treated by addition of 56 ml of 30% hydrogen peroxide and 1.0 g of osmium tetroxide in 80 ml of trimethylcarbinol. After the mixture has stood at room temperature for 22 h, 12 ml of hydrogen peroxide are added. The reaction mixture is allowed to stand at room temperature for an additional 20 h, then concentrated in vacuo to 1/3 of its original volume, diluted with water, and the reaction product extracted with ethyl acetate. The extract is washed with water, several times with 10% sodium bisulfite solution, then with 4% sodium bicarbonate solution and finally with water to neutrality. The residue remaining after evaporation of the solvent does not show ultraviolet absorption. 1.0 g of this crude substance, by crystallization from methanol, gives l7αmethylandrostane-4,5,17β-triol-3-one (0.400 g) melting at 192°-194°C.
A solution of 20.0 g of crude 17β-methylandrostane-4,5,17β-triol-3-one in 1 L of methanol is heated under reflux in a stream of nitrogen for 20 min; then 20.0 g of potassium hydroxide in 40 ml of water and 200 ml of methanol are added. 5 min after the addition, the solution is treated by addition of 20 ml of acetic acid and concentrated in vacuo. The residue is diluted with water containing sodium chloride and extracted three times with ethyl acetate. The extract is washed with 10% sodium bicarbonate solution and then with water to neutrality. The residue remaining after evaporation of the solvent is
dissolved in acetone; addition of petroleum ether gives 4-hydroxy-17αmethyl-testosterone (8.0 g) melting at 168°-170°C. The mother liquors chromatographed over Florisil 30-60 mesh yield an additional 5.0 g of the same substance melting at 168°-170°C.
Therapeutic Function
Anabolic, Androgen
Oxymesterone Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
Oxymesterone Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 50000 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| LGM Pharma | 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2127 | 70 |
| Aishilun biotechnology (Shanghai) co., LTD | 021-50676523 18019098996 | info@acelybio.com | China | 3967 | 58 |
| Supplier | Advantage |
|---|---|
| CONIER CHEM AND PHARMA LIMITED | 58 |
| GIHI CHEMICALS CO.,LIMITED | 58 |
| Mainchem Co., Ltd. | 55 |
| LGM Pharma | 70 |
| Aishilun biotechnology (Shanghai) co., LTD | 58 |