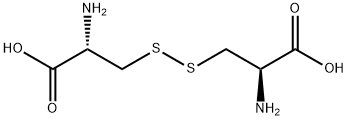
L-Cystine
- CAS No.
- 56-89-3
- Chemical Name:
- L-Cystine
- Synonyms
- (H-CYS-OH)2;(CYS)2;l-cystin;-Cystine;1-cystine;Cystine CRS;Cystine acid;L-(-)-CYSTINE;L-CYSTINE BASE;(r-(r*,r*))-3,3’-dithiobis(2-aminopropanoicacid)
- CBNumber:
- CB6394079
- Molecular Formula:
- C6H12N2O4S2
- Molecular Weight:
- 240.3
- MDL Number:
- MFCD00064228
- MOL File:
- 56-89-3.mol
| Melting point | >240 °C (dec.) (lit.) |
|---|---|
| Boiling point | 468.2±45.0 °C(Predicted) |
| alpha | -224 º(c=2 in 1M HCl) |
| Density | 1.68 |
| refractive index | -222.5 ° (C=1, 1mol/L HCl) |
| storage temp. | 2-8°C |
| solubility | 1 M HCl: 100 mg/mL |
| form | Powder/Solid |
| pka | 1.0, 2.1, 8.02, 8.71(at 25℃) |
| color | White |
| PH | 4.60 |
| optical activity | [α]20/D 219±5°, c = 1% in 1 M HCl |
| Water Solubility | 0.112 g/L (25 ºC) |
| Merck | 14,2782 |
| BRN | 1728094 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | LEVWYRKDKASIDU-IMJSIDKUSA-N |
| LogP | 0.773 (est) |
| CAS DataBase Reference | 56-89-3(CAS DataBase Reference) |
| Substances Added to Food (formerly EAFUS) | L-CYSTINE |
| FDA 21 CFR | 172.320 |
| FDA UNII | 48TCX9A1VT |
| NIST Chemistry Reference | L-Cystine(56-89-3) |
| EPA Substance Registry System | L-Cystine (56-89-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-22 | |||||||||
| Safety Statements | 26-36-24/25 | |||||||||
| RIDADR | 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | HA2690000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29309014 | |||||||||
| NFPA 704 |
|
L-Cystine price More Price(77)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SAB1305544 | MONOCLONAL ANTI-NEPHRIN antibody produced in mouse clone 174CT2.1.1, crude ascites, buffered aqueous solution | 56-89-3 | 100μL | $466 | 2024-03-01 | Buy |
| Sigma-Aldrich | 30200 | L-Cystine ≥99.7% (TLC) | 56-89-3 | 25g | $43.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 2470 | L-Cystine - CAS 56-89-3 - Calbiochem | 56-89-3 | 25G | $77.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1161586 | Cystine United States Pharmacopeia (USP) Reference Standard | 56-89-3 | 200mg | $519 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02836 | L-Cystine suitable for biopharmaceutical production FCC | 56-89-3 | 1kg | $305 | 2024-03-01 | Buy |
L-Cystine Chemical Properties,Uses,Production
Description
L-cystine (formula: (SCH2CH(NH2) CO2H)2), the L-form of cystine) is a covalently linked dimeric nonessential amino acid formed through the oxidation of cysteine. It is contained in many foods including eggs, meat, dairy products, and whole grains as well as in skin and hairs. L-cystine and L-methionine are the amino-acids required for wound healing and formation of epithelial tissue. It is able to stimulate the hematopoietic system and promote the formation of white and red blood cells. It can also be used as a component of parental and enteral nutrition. It can also be used for the treatment of dermatitis and protection of liver function. L-cystine is manufactured through the enzymatic conversion from DL-amino thiazoline carboxylic acid.
References
http://www.ajiaminoscience.com/products/manufactured_products/l-amino_acids/L-Cystine.aspx
https://pubchem.ncbi.nlm.nih.gov/compound/L-cystine#section=Pharmacology
https://en.wikipedia.org/wiki/Cystine
Chemical Properties
A white or almost white, crystalline powder, practically insoluble in water and in alcohol.
Uses
L-Cystine is a non-essential amino acid for human development. L-Cystine is formed by the dimerization of two cysteines through the sulfur.
Uses
L-Cystine is used as an antioxidant, protecting tissues against radiation and pollution. It finds application in protein synthesis. It is required for utilization of vitamin B6 and is useful in healing burns and wounds. It is also is required by certain malignant cell lines in the culture medium as well as for the growth of certain micro-organisms. It is useful in the stimulation of hematopoietic system and promotes the formation of white and red blood cells. It is an active ingredient in medications used to treat dermatitis.
Uses
L-Cystine has been used in in vitro cystine solubility assay to identify potential drugs that influence cystine solubility. It is also used as a supplement of phosphate-buffered saline to slice and wash periprosthetic tissues.
Definition
ChEBI: L-cystine is the L-enantiomer of the sulfur-containing amino acid cystine. It has a role as a flour treatment agent, a human metabolite, a Saccharomyces cerevisiae metabolite, a mouse metabolite and an EC 1.2.1.11 (aspartate-semialdehyde dehydrogenase) inhibitor. It is a cystine, a L-cysteine derivative and a non-proteinogenic L-alpha-amino acid. It is a conjugate acid of a L-cystine anion. It is an enantiomer of a D-cystine. It is a tautomer of a L-cystine zwitterion.
General Description
This Standard Reference Material (SRM) is intended primarily for use in validating microchemical procedures for the determination of carbon, hydrogen, nitrogen, and sulfur in organic matter. SRM 143d is pure crystalline cystine supplied in a 2 g unit size. For more information, please refer to the SDS and COA.
SRM 143D_cert SRM 143D _SDS
Biochem/physiol Actions
Cysteine is the source of disulfide linkages in proteins and has a role in sulfur transport. It undergoes rapid oxidation to form cystine at physiological pH. L-cystine is crucial for oxygen production and low density lipoprotein modification by arterial smooth muscle cells. It also has a role in the synthesis of glutathione.
Safety Profile
Low toxicity by ingestion. When heated to decomposition it emits toxic fumes of PO, and SOx
Purification Methods
Cystine disulfoxide impurity is removed by treating an aqueous suspension with H2S. The cystine is filtered off, washed with distilled water and dried at 100o under a vacuum over P2O5. Crystallise it by dissolving in 1.5M HCl, then adjusting to neutral pH with ammonia. Likely impurities are D-cystine, meso-cystine and tyrosine. Also purify it by dissolving it in 10% NH3 and adding gradually dilute AcOH until the point of precipitation and cooling slowly [Dughton & Harrison Acta Cryst 12 396, 402 1959.] Alternatively dissolve it in 6N NH4OH and evaporate it at room temperature for crystallisation to occur. [Chaney & Steinrauf Acta Cryst 30 711 1974, Beilstein 4 IV 3155.]
L-Cystine Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| DONBOO AMINO ACID COMPANY | +8613063595538 | donboo@donboo.com | China | 9363 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8816 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 2990 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20288 | 58 |
| Shandong Juchuang Chemical Co., LTD | +undefined15030412209 | admin@juchuangchem.com | China | 387 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 | admin@hbsaisier.cn | China | 1015 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 +86-18633929156 | admin@hblongbang.com | China | 941 | 58 |
Related articles
- L-Cystine: Metabolism and Biological Functions
- L-Cystine, a sulfur-containing amino acid, crucial for protein folding, synthesis of taurine, sulfate, and glutathione, essent....
- Jun 13,2024
View Lastest Price from L-Cystine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-10 | L-Cystine
56-89-3
|
US $3.50 / kg | 1kg | ≥99% | 3000tons/month | Hebei Andu Technology Com.,Ltd | |
 |
2024-11-09 | L-Cystine
56-89-3
|
US $999.00-800.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-09 | L-Cystine
56-89-3
|
US $41.00 / mg | 99.85% | 10g | TargetMol Chemicals Inc. |