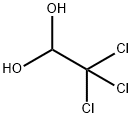
포수클로랄
|
|
포수클로랄 속성
- 녹는점
- 57 °C(lit.)
- 끓는 점
- 97 °C
- 밀도
- 1.43 g/mL at 20 °C
- 증기압
- 19.998hPa at 25℃
- 굴절률
- 1.4603 (estimate)
- 인화점
- 16 °C
- 저장 조건
- 0-6°C
- 용해도
- 물에 매우 잘 녹고 에탄올에 잘 녹습니다(96%).
- 산도 계수 (pKa)
- 10(at 25℃)
- 물리적 상태
- 결정성 고체
- Specific Gravity
- 1.91
- 수소이온지수(pH)
- 3.5-5.5 (20℃, 10%)
- 수용성
- 660g/100mL
- Merck
- 13,2080
- BRN
- 1698497
- Dielectric constant
- 5.5(15℃)
- 안정성
- 안정적이지만 공기나 빛에 민감할 수 있습니다. 알코올, 시안화물, 요오드, 강염기, 탄산염과 호환되지 않습니다.
- InChIKey
- RNFNDJAIBTYOQL-UHFFFAOYSA-N
- LogP
- 1.092 at 25℃
- CAS 데이터베이스
- 302-17-0(CAS DataBase Reference)
- IARC
- 2A (Vol. 63, 84, 106) 2014
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,F,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 25-36/38-39/23/24/25-23/24/25-11-36/37/38-36-20/21/22 | ||
| 안전지침서 | 26-45-25-23-36/37-16-27 | ||
| 유엔번호(UN No.) | UN 3286 3/PG 2 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | FM8750000 | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | III | ||
| HS 번호 | 29055900 | ||
| 유해 물질 데이터 | 302-17-0(Hazardous Substances Data) | ||
| 독성 | LD50 oral in rat: 479mg/kg | ||
| 기존화학 물질 | KE-34070 |
포수클로랄 C화학적 특성, 용도, 생산
개요
Barbiturates와는 달리 효소 유도 효과를 지니지 않으며 REM 수면에 영향을 미치지 않는다. 또한 상용량에서는 호흡, 혈압, 반사 등에 거의 영향을 미치지 않으며, 숙취효과도 barbiturates보다 적다.용도
수면진정제 및 신경안정제.개요
Chloral hydrate is one of the oldest sedatives used for dental sedation. It was first synthesized in 1832 by Justus von Liebig and was the first synthetic central nervous system (CNS) depressant. It was used to treat delirium tremens, insomnia, and anxiety, although it is considered an unapproved drug by the US Food and Drug Administration. Initially considered to be a safer alternative to opium, it was noted to produce rapid unconsciousness when combined with ethanol. Physical dependence can occur with chronic use.Chloral hydrate is classified as a sedative-hypnotic and is known to induce sleep in children. It has been very popular in pediatric dentistry since the mid-1950s. Chloral hydrate is rapidly absorbed following oral administration and is converted through its first pass in the liver to trichloroethanol,its active form. Trichloroethanol is conjugated in the liver and excreted in the urine. Like other agents that are metabolized in the liver, chloral hydrate may interact with other drugs, herbs, or foods resulting in clinically significant alterations of the agents (e.g.,warfarin).
용도
Trichloroacetaldehyde Hydrate is a useful chemical reagent used as a sedative/hypnotic agent for the short-term treatment of insomnia. First developed in 1832, chloral hydrate is the oldest sleep medication still in use today. This medication is also used to calm you just before surgery or other procedures. It works by affecting certain parts of the brain to cause calmness.Studies have shown that when used in pediatric sedation side effects such as hallucination, excessive sleep and seizures were observed. Drowsiness and trouble waking up in the morning, nausea, vomiting, stomach pain, diarrhea, and headache may occur. Stomach problems can be reduced by taking chloral hydrate with a full glass of water. It is sometimes administered to patients being treated with cyclophosphamide and it is known to inhibit some aldehyde dehydrogenases.
Besides, Chloral hydrate is a starting point for the synthesis of other organic compounds. It is the starting material for the production of chloral, which is produced by the distillation of a mixture of chloral hydrate and sulfuric acid, which serves as the desiccant.
정의
ChEBI: Chloral hydrate is an organochlorine compound that is the hydrate of trichloroacetaldehyde. It has a role as a sedative, a general anaesthetic, a mouse metabolite and a xenobiotic. It is an organochlorine compound, an aldehyde hydrate and an ethanediol.Biological Functions
Chloral hydrate (Noctec, Somnos) was developed in the late 1800s and is still used as a sedative–hypnotic agent. It is a hydrated aldehyde with a disagreeable smell and taste that is rapidly reduced in vivo to trichloroethanol, which is considered to be the active metabolite. It produces a high incidence of gastric irritation and allergic responses, occasionally causes cardiac arrhythmias, and is unreliable in patients with liver damage.일반 설명
Chloral hydrate, trichloroacetaldehydemonohydrate, CCl3CH(OH)2 (Noctec), is analdehyde hydrate stable enough to be isolated. The relativestability of this gem-diol is largely a result of an unfavorabledipole–dipole repulsion between the trichloromethyl carbonand the carbonyl carbon present in the parent carbonylcompound.Chloral hydrate is unstable in alkaline solutions, undergoingthe last step of the haloform reaction to yield chloroformand formate ion. In hydroalcoholic solutions, it formsthe hemiacetal with ethanol. Whether or not this compoundis the basis for the notorious and potentially lethal effect of the combination of ethanol and chloral hydrate (the “MickeyFinn”) is controversial. Synergism between two differentCNS depressants also could be involved. Additionally,ethanol, by increasing the concentration of nicotinamideadenine dinucleotide (NADH), enhances the reduction ofchloral to the more active metabolite trichloroethanol, andchloral can inhibit the metabolism of alcohol because it inhibitsalcohol dehydrogenase. Chloral hydrate is a weak acidbecause its CCl3 group is very strong electron withdrawing.A 10% aqueous solution of chloral hydrate has pH 3.5 to4.4, which makes it irritating to mucous membranes in thestomach. As a result, GI upset commonly occurs for thedrug if undiluted or taken on an empty stomach. Chloral hydrateas a capsule, syrup, or suppository is currently available.
공기와 물의 반응
Water soluble.반응 프로필
Chloral hydrate is incompatible with alkalis, alkaline earth metals, alkali carbonates and soluble barbiturates. Chloral hydrate is decomposed by sodium hydroxide. Chloral hydrate reduces ammoniacal silver nitrate. Chloral hydrate liquefies when triturated with an equal quantity of menthol, camphor or thymol. . Reaction of Chloral hydrate with hydroxylamine produces toxic hydrogen cyanide gas, Org. Synth., 1941, Vol. 1, 377.위험도
Overdose toxic, hypnotic drug, dangerous to eyes. Probable carcinogen.화재위험
Flash point data for Chloral hydrate are not available; however, Chloral hydrate is probably combustible.Clinical Use
Although it is suggested that chloral hydrate per semay act as a hypnotic,chloral hydrate is very quickly convertedto trichloroethanol, which is generally assumed toaccount for almost all of the hypnotic effect. Thetrichloroethanol is metabolized by oxidation to chloral andthen to the inactive metabolite, trichloracetic acid, which is also extensively metabolized to acylglucuronidesvia conjugation with glucuronic acid. It appears tohave potent barbiturate-like binding to GABAAreceptors.Although an old drug, it still finds use as a sedative in nonoperatingroom procedures for the pediatric patient.Safety Profile
A human poison by ingestion and possibly other routes. Poison experimentally by ingestion, intravenous, and rectal routes. Moderately toxic by subcutaneous, parenteral, and intraperitoneal routes, Experimental reproductive effects. Human systemic effects by ingestion: general anesthetic, cardiac arrhythmias, blood pressure depression, eye effects, coma, pulse rate increase, arrhythmias. Human mutation data reported. Questionable carcinogen with experimental carcinogenic and tumorigenic data by skin contact. A sedative, anesthetic, and narcotic. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of Cl-.잠재적 노출
Chloral is used as an intermediate in the manufacture of such pesticides as DDT, methoxychlor, DDVP, naled, trichlorfon, and TCA. Chloral is also used in the production of chloral hydrate; used as a therapeutic agent with hypnotic, sedative, and narcotic effects; used in a time prior to the introduction of barbituratesCarcinogenicity
Chloral hydrate has not been adequately tested for teratogenicity, reproductive effects, or chronic toxicity. Similarly, no histological evaluations have been conducted.환경귀착
Chloral hydrate is a CNS depressant, but its mechanism of action is not well known. Coingestion with ethanol produces enhanced effects by several mechanisms. First, ethanol competes for alcohol and aldehyde dehydrogenase, which then prolongs the half-life of ethanol. The metabolism of ethanol generates the reduced form of NADH, which is a cofactor for the metabolism of chloral hydrate to its active metabolite trichloroethanol. Finally, ethanol inhibits the conjugation of trichloroethanol to its inactive form urochloralic acid. This results in enhanced CNS depression.운송 방법
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.비 호환성
Chloral hydrate reacts with strong bases forming chloroform. Contact with acids, or exposure to light may cause polymerization. Reacts with water, forming chloral hydrate. Reacts with oxidizers, with a risk of fire or explosions.폐기물 처리
Incineration after mixing with another combustible fuel; care must be taken to assure complete combustion to prevent phosgene formation; an acid scrubber is necessary to remove the halo acids produced.포수클로랄 준비 용품 및 원자재
원자재
준비 용품
클로랄
5-Methoxy-3-indazolecarboxylic acid
Tenidap
Etodolac
6-Bromoisatin
1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE
5-Methoxyisatin
2-AMINO-5,6-DICHLOROBENZOIC ACID
트리클로르아세트 산
다이클로로다이페닐트라이클로로에테인(디클로로디페닐트리클로로에탄)
2-Oxiranecarboxylicacid, 3-(trichloromethyl)-, ethyl ester
5-Chloroisatin
DCU
1H,1H,9H-Hexadecafluoro-1-nonanol
4,7-DIFLUOROISATIN
5,6-difluoro-indoline-2,3-dione
2,2,3,3,4,4,4-Heptafluoro-1-butanol
포수클로랄 공급 업체
글로벌( 529)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| shandong exelon biochem co.ld | +86-533-2119995 +8615053330977 |
451614802@QQ.com | China | 28 | 58 |
| Wuhan Kemi-Works Chemical Co., Ltd. | +86-2785736489 +8618171418573 |
sales@kemiworks.com | China | 704 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12471 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | +86-86-18838029171 +8618126314766 |
ada@sh-teruiop.com | China | 893 | 58 |
| Zibo Wei Bin Import & Export Trade Co. Ltd. | +86-0533-2091136 +8613864437655 |
ziboweibinmaoyi@163.com | China | 100 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 18229 | 58 |
| Sigma Audley | +86-18336680971 +86-18126314766 |
nova@sh-teruiop.com | China | 525 | 58 |
| Shanghai Aosiris new Material Technology Co., LTD | 86-15139564871 +8615139564871 |
wrjmoon2000@163.com | China | 357 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 1125 | 58 |