세바식산
|
|
세바식산 속성
- 녹는점
- 133-137 °C (lit.)
- 끓는 점
- 294.5 °C/100 mmHg (lit.)
- 밀도
- 1.21
- 증기압
- 1 mm Hg ( 183 °C)
- 굴절률
- 1.422
- 인화점
- 220 °C
- 저장 조건
- Store below +30°C.
- 용해도
- 에탄올: 100mg/mL
- 물리적 상태
- 분말 또는 과립
- 산도 계수 (pKa)
- 4.59, 5.59(at 25℃)
- 색상
- 흰색에서 황백색까지
- 냄새
- 단클린 프리즘 정제, 흰색. 분말, 지방산 냄새.
- 수용성
- 1g/L(20℃)
- Merck
- 14,8415
- BRN
- 1210591
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제, 염기, 환원제와 호환되지 않습니다.
- InChIKey
- CXMXRPHRNRROMY-UHFFFAOYSA-N
- LogP
- 1.5 at 23℃
- CAS 데이터베이스
- 111-20-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36-24/25 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | VS0875000 | ||
| TSCA | Yes | ||
| HS 번호 | 29171310 | ||
| 독성 | LD50 orally in Rabbit: 3400 - 14500 mg/kg LD50 dermal Rat > 2000 mg/kg | ||
| 기존화학 물질 | KE-09402 |
세바식산 C화학적 특성, 용도, 생산
개요
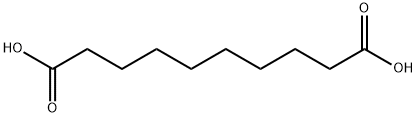
Sebacic acid is a dicarboxylic acid with structure (HOOC)(CH2)8(COOH), and is naturally occurring.In its pure state it is a white flake or powdered crystal. The product is described as non-hazardous, though in its powdered form it can be prone to flash ignition (a typical risk in handling fine organic powders).
Sebaceus is Latin for tallow candle, sebum (tallow) is Latin for tallow, and refers to its use in the manufacture of candles. Sebacic acid is a derivative of castor oil, with the vast majority of world production occurring in China which annually exports over 20,000 metric tonnes, representing over 90 % of global trade of the product.
In the industrial setting, sebacic acid and its homologues such as azelaic acid can be used in plasticizers, lubricants, hydraulic fluids, cosmetics, candles, etc. Sebacic acid is also used as an intermediate for aromatics, antiseptics, and painting materials.
화학적 성질
White flaky crystals. Slightly soluble in water, soluble in alcohol and ether.용도
Decanedioic acid was named by Thenard LJ (1802) from the Latin sebaceus(tallow candle) or sebum (tallow) in reference to its use in the manufacture of candles. Thenard LJ isolated this compound from distillation products of beef tallow. In 1954, it was reported that it was produced in excess of 10,000 tons annually by alkali fission of castor oil. Sebacic acid and its derivatives, as azelaic acid, have a variety of industrial uses as plasticizers, lubricants, diffusion pump oils, cosmetics, candles, etc. It is also used in the synthesis of polyamide, as nylon, and of alkyd resins. An isomer, isosebacic acid, has several applications in the manufacture of vinyl resin plasticizers, extrusion plastics, adhesives, ester lubricants, polyesters, polyurethane resins and synthetic rubber.정의
ChEBI: Sebacic acid is an alpha,omega-dicarboxylic acid that is the 1,8-dicarboxy derivative of octane. It has a role as a human metabolite and a plant metabolite. It is an alpha,omega-dicarboxylic acid and a dicarboxylic fatty acid. It is a conjugate acid of a sebacate(2-) and a sebacate. It derives from a hydride of a decane.제조 방법
Sebacic acid is normally made from castor oil, which is essentially glycerol triricinoleate. The castor oil is heated with sodium hydroxide at about 250??e. This treatment results in saponification of the castor oil to ricinoleic acid which is then cleaved to give 2-octanol and sebacic acid: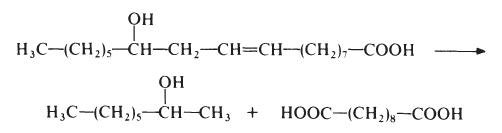
This process results in low yields of sebacic acid (about 50% based on the castor oil) but, nevertheless, other routes have not proved competitive. Sebacic acid is a colourless crystalline solid, m.p. 134??.
일반 설명
White granular powder. Melting point 153°F. Slightly soluble in water. Sublimes slowly at 750 mm Hg when heated to melting point.공기와 물의 반응
Insoluble in water.반응 프로필
Sebacic acid reacts exothermically to neutralize bases, both organic and inorganic. May react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Can react with active metals to form gaseous hydrogen and a metal salt. Such reactions are slow in the dry, but systems may absorb enough water from the air to allow corrosion of iron, steel, and aluminum parts and containers. Reacts slowly with cyanide salts to generate gaseous hydrogen cyanide. Reacts with solutions of cyanides to cause the release of gaseous hydrogen cyanide. May generate flammable and/or toxic gases and heat with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. May react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Can be oxidized exothermically by strong oxidizing agents and reduced by strong reducing agents. May initiate polymerization reactions.화재위험
Flash point data for Sebacic acid are not available. Sebacic acid is probably combustible.Purification Methods
Purify sebacic acid via the disodium salt which, after crystallisation from boiling water (charcoal), is again converted to the free acid. The free acid is crystallised repeatedly from hot distilled water or from Me2CO/pet ether and dried under vacuum. [Beilstein 2 IV 2078.]세바식산 준비 용품 및 원자재
원자재
LACQUER THINNER
Solvent oil No.200
Azelaic acid disodium salt
소듐캐스터레이트
피마자유
N-데칸
사염화탄소
사이클로펜텐
수산화나트륨
Sebacic acid monosodium salt
사이클로펜타논
Monoester
황산
그리스
준비 용품
옥타코산산
nylon 1010
DL-2-옥탄올
다이에틸세바케이트
디옥틸 세바케이트
1,10-데칸디올
SEBACIC ACID DI-N-OCTYL ESTER
Sebaconitrile
14-METHYLPENTADECANOIC ACID
디메틸 세바케이트
디카프릴 프탈산
1-PYRENEDECANOIC ACID
Diisooctyl sebacate
디부틸 세바케이트
Cleaning agent
METHYL 10-HYDROXYDECANOATE
1,4-dioxacyclotetradecane-5,14-dione
옥타디인(1,7-)
SEBACIC DIHYDRAZIDE
세바식산 공급 업체
글로벌( 703)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hengshui Haoye Chemical Co.,Ltd. | +86-2102300 +86-18632882519 |
hy@chemcoms.com | China | 256 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3050 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 6008 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 986 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 18226 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 1856 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
forrest.lee@dakenchem.com | China | 15956 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
세바식산 관련 검색:
대두 기름, 에폭시화된 이산화티타늄 메틸 비닐 에테르-말레 무수물 공중합체 디아이소프로필 세바케이트 2,5-퓨란디온, 텔로머TELO머 ,함유 에텐일벤젠 AND (1-메틸에틸) 벤젠 디나트륨세박산 세바식산 말레산, 중합체, 메틸 비닐 에테르 함유 디부틸 세바케이트 디메틸 세바케이트 디옥틸 세바케이트 디옥틸 호박산염 비스(2,2,6,6-테트라메틸-4-피페리디닐)세바크산 세바코일 염화물 다이리놀레익애씨드/세바식애씨드/피페라진/에틸렌다이아민코폴리머
AZELAIC ACID MONOMETHYL ESTER
Suberic acid
Pimelic acid