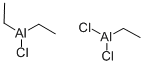에틸알미늄세스키클로라이드 C화학적 특성, 용도, 생산
화학적 성질
clear colorless solution
용도
Catalyst for olefin polymerization, aromatic
hydrogenation; intermediate.
생산 방법
Ethyl chloride and aluminum could used as starting metarials to synthesize Ethylaluminum sesquichloride. A 9.5 × 10
-2 m
3 closed steel reactor, fitted with a reflux condenser and equipped for vacuum distillation of product, was first purged with nitrogen. After addition of 13.6 kg of aluminum powder and a catalyst mixture composed of 3.6 kg diethylaluminum chloride and 410 g iodine, the reactor contents were stirred and heated slowly to 130 ℃. The temperature was maintained at 120 – 150 ℃ during addition of 50 kg ethyl chloride over a period of 3 h. The product can be flash distilled under vacuum, or it can be drained from the reactor and clarified by settling or by filtration of black residual metal solids. Iodine can be omitted from the procedure, in which case the induction period may be increased.
일반 설명
A clear yellow liquid. Slightly denser than water. Used to make other chemicals.
공기와 물의 반응
Spontaneously flammable in air (Douda 1966). Reacts violently with water forming hydrogen chloride and flammable ethane gas (Rose 1961).
반응 프로필
Organometallics, such as ETHYLALUMINUM SESQUICHLORIDE, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. Organometallics containing halogens (fluorine, chlorine, bromine, iodine) bonded to the metal typically with generate gaseous hydrohalic acids (HF, HCl, HBr, HI) with water. A mixture of ETHYLALUMINUM SESQUICHLORIDE with carbon tetrachloride exploded when warmed to room temperature [Bretherick, 5th Ed., 1995].
위험도
Ignites on contact with air, dangerous fire
risk, reacts violently with water.
건강위험
Inhalation of smoke from fire causes metal-fume fever (flu-like symptoms); acid fumes irritate nose and throat. Contact with liquid, which is spontaneously flammable, causes severe burns of eyes and skin.
Safety Profile
Mixtures with carbon
tetrachloride explode at room temperature.
When heated to decomposition it emits
toxic fumes of Cl-. See also ALUMINUM
COMPOUNDS.
잠재적 노출
These materials are used as components of olefin polymerization catalysts. The reader is referred to the entry on “Aluminum alkyls” for additional information on this entry. The aluminum alkyl halides parallel very closely the aluminum alkyls
운송 방법
UN3052 Spontaneously combustible. Water reactive releasing large quantities of toxic and deadly hydrogen gas. (Note: this number does not appear in the 49/CFR HazMat tables)
비 호환성
The aluminum alkyl halides are strong reducing agents; they react—possibly violently—with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. These chemicals react violently with nitromethaneEthylaluminum sesquichloride reacts explosively with carbon tetrachloride at room temperature. This chemical reacts violently with water, forming corrosive hydrogen chloride and flammable ethane gas. Diethylaluminum chloride may form an explosive product with chlorine azide.
에틸알미늄세스키클로라이드 준비 용품 및 원자재
원자재
준비 용품