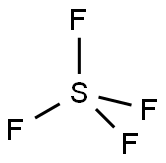
테트라플루오린화 황(테트라플루오르화 황) C화학적 특성, 용도, 생산
화학적 성질
Sulfur tetrafluoride is a colorless gas with an odor like sulfur dioxide. Shipped as a liquefied compressed gas.
용도
Compounds of interest in this group include sulfur hexafluoride (SF6) used as an electrical insulating material in circuit breakers, cables, capacitors, and transformers, and its degradation products, which are produced when electrical arcing occurs. The specific compounds produced depend on the arcing conditions. In anaerobic and anhydrous circumstances, sulfur tetrafluoride (SF4) is produced; if moisture is present, the tetrafluoride may hydrolyze to form thionyl fluoride (SOF2) and HF. Sulfuryl fluoride (SO2F2), disulfur decafluoride (S2F10), sulfur pentafluoride (SF5), and sulfur dioxide are also formed from sulfur hexafluoride. Some of these compounds are also produced as contaminants in the commercial production of sulfur hexafluoride by burning sulfur in fluorine gas, for example, sulfur tetrafluoride and disulfur decafluoride, as well as sulfur monofluoride (S2F2).
일반 설명
Sulfur tetrafluoride is a colorless gas with a distinct sulfur odor. Sulfur tetrafluoride is highly toxic by inhalation and a strong irritant to skin, eyes and mucous membranes. Sulfur tetrafluoride reacts vigorously with water and acids to yield toxic fluoride and sulfur oxide fumes and an acidic solution. Sulfur tetrafluoride is heavier than air. Under prolonged exposure to fire or intense heat the containers may violently rupture or rocket. Sulfur tetrafluoride is used as a fluoridizing agent and as an oil repellent.
공기와 물의 반응
Violent reaction with water. Sulfur tetrafluoride reacts vigorously with water and acids to yield toxic fluoride and sulfur oxide fumes and an acidic solution.
반응 프로필
Sulfur tetrafluoride is a highly toxic and corrosive gas. On contact with water, steam, or mineral acids Sulfur tetrafluoride decomposes and produces toxic and highly irritating fumes. When heated to decomposition Sulfur tetrafluoride emits very toxic fluoride and sulfur oxides fumes [Lewis, 3rd ed., 1993, p. 1197]. Explosively violent reactions with 2-(hydroxymethyl)furan or 2-methyl-3-butyn-2-ol even below -50° C have been recorded [Bretherick, 5th ed., 1995, p. 1432]. Ignition or explosion may occur on contact with dioxygen difluoride even below -100° C [Streng, A. G., Chem. Rev., 1963, 63, p. 615].
위험도
High by inhalation, strong irritant to eyes,
mucous membranes, and upper respiratory tract irritant.
Lung damage.
건강위험
Sulfur tetrafluoride is highly toxic by inhalation; it is a strong irritant to eyes and mucous membranes. Poisonous; may be fatal if inhaled. Contact may cause burns to skin and eyes. Contact with liquid may cause frostbite.
화재위험
Container may explode in heat of fire. When heated to decomposition, Sulfur tetrafluoride emits very toxic fumes of fluorides and sulfur oxides. Reacts violently with water. Sulfur tetrafluoride is decomposed by concentrated sulfuric acid. Thermostable to 1112F.
Safety Profile
Poison by inhalation. A powerful irritant. Will react with water, steam, or acids to yield toxic and corrosive fumes. Incompatible with dioxygen difluoride. When heated to decomposition it emits very toxic fumes of Fand SOx. See also FLUORIDES.
잠재적 노출
Sulfur tetrafluoride is used as a selective fluorinating agent in making water-repellent and oil
repellent materials and lubricity improvers. It is also used as a pesticide intermediate.
운송 방법
UN2418 Sulfur tetrafluoride, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 8-Corrosive material, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. Forbidden to be transported by any aircraft or by rail tank car.
비 호환성
Keep away from moisture, concentrated sulfuric acid, dioxygen difluoride. Reacts vigorously with water, alcohols and acids releasing toxic fluoride, sulfur oxide fumes and forming a corrosive acid solution. Readily hydrolyzed by moisture, forming hydrofluoric acid, thionyl fluoride. Attacks glass, ceramic, concrete
폐기물 처리
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillabletype cylinders should be returned to original supplier with any valve caps and outlet plugs secured and valve protection caps in place.
테트라플루오린화 황(테트라플루오르화 황) 준비 용품 및 원자재
원자재
준비 용품